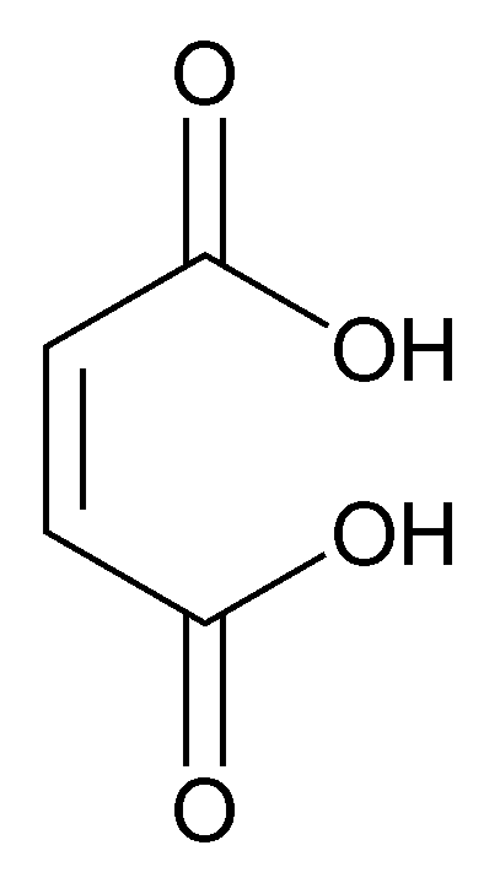
Maleic Acid
» Maleic Acid contains not less than 99.0 percent and not more than 101.0 percent of C4H4O4, calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight glass containers, protected from light. Store at room temperature.
Identification—
A:
Dissolve about 500 mg of Maleic Acid in 10 mL of water: the pH of the solution is less than 2.
B:
The principal spot in the chromatogram obtained from Test solution 2 corresponds in color, size, and RF value to that in the chromatogram obtained from the Maleic acid standard solution, as obtained in the test for Limit of fumaric acid.
C:
Dissolve about 35 mg of resorcinol in 10 mL of sulfuric acid (Resorcinol solution). Dissolve about 100 mg of Maleic Acid in 10 mL of water (Test solution). To 0.3 mL of the Test solution add 3 mL of the Resorcinol solution, and heat in a water bath for 15 minutes: no color develops. To 3 mL of the Test solution add 1 mL of bromine TS, heat in a water bath for 15 minutes to remove the bromine, then heat to boiling, and cool. To 0.2 mL of this solution, add 3 mL of the Resorcinol solution, and heat in a water bath for 15 minutes: a violet-pink color develops.
Color and clarity of solution—
Dilute hydrochloric acid solution—
Mix 27.5 mL of hydrochloric acid with sufficient water to make 1000 mL.
Reference solution—
Mix 2.4 mL of ferric chloride CS and 0.6 mL of cobaltous chloride CS with Dilute hydrochloric acid solution to make 10 mL. Dilute 5 mL of this solution with Dilute hydrochloric acid solution to make 100 mL.
Test solution—
Dissolve about 5 g of Maleic Acid in 50 mL of water.
Procedure—
Place the Reference solution and the Test solution in matched color-comparison tubes, and compare the solutions by viewing them downward against a white surface (see Color and Achromicity  631
631 ): the Test solution is clear and not more intensely colored than the Reference solution.
): the Test solution is clear and not more intensely colored than the Reference solution.
Water, Method I  921
921 :
not more than 2.0% is found.
:
not more than 2.0% is found.
Residue on ignition  281
281 :
not more than 0.1%, determined on a 1.0-g portion.
:
not more than 0.1%, determined on a 1.0-g portion.
Heavy metals, Method II  231
231 —
—
Test solution—
Transfer 1.0 g of Maleic Acid to a quartz crucible, add 0.5 g of magnesium oxide, and mix. Ignite the crucible to dull redness until a homogeneous white or grayish-white mass is obtained. Ignite at 800 for 1 hour, cool, and dissolve the residue by adding two 5-mL portions of diluted hydrochloric acid. Add 0.1 mL of phenolphthalein TS, and then add ammonium hydroxide until a pink color is obtained. Cool, add glacial acetic acid until the solution is decolorized, then add 0.5 mL of glacial acetic acid in excess, and dilute with water to 20.0 mL.
for 1 hour, cool, and dissolve the residue by adding two 5-mL portions of diluted hydrochloric acid. Add 0.1 mL of phenolphthalein TS, and then add ammonium hydroxide until a pink color is obtained. Cool, add glacial acetic acid until the solution is decolorized, then add 0.5 mL of glacial acetic acid in excess, and dilute with water to 20.0 mL.
Standard solution—
To 0.5 g of magnesium oxide, add 1.0 mL of Standard Lead Solution, and evaporate to dryness at 105 for 1 hour. Following the procedure described above for preparation of the Test solution, ignite, dissolve in diluted hydrochloric acid, add ammonia and then acetic acid, and dilute with water to 20.0 mL.
for 1 hour. Following the procedure described above for preparation of the Test solution, ignite, dissolve in diluted hydrochloric acid, add ammonia and then acetic acid, and dilute with water to 20.0 mL.
Procedure—
To 12 mL of the Test solution, add 2.0 mL of pH 3.5 Acetate Buffer, mix, add to 1.2 mL of thioacetamide–glycerin base TS, and mix immediately. To 10 mL of the Standard solution, add 2.0 mL of the Test solution and 2.0 mL of pH 3.5 Acetate Buffer, mix, add 1.2 mL of thioacetamide–glycerin base TS, and mix immediately. Prepare a blank, using a mixture of 10 mL of water and 2.0 mL of the Test solution. Compared to the blank, the solution obtained from the Standard solution shows a light brown color. Dilute each of the solutions obtained from the Test solution and the Standard solution with water to 50 mL, allow to stand for 2 minutes, and view downward over a white surface. The color of the solution obtained from the Test solution is not darker than that of the solution obtained from the Standard solution: not more than 10 µg per g.
Limit of fumaric acid—
Adsorbent:
0.25-mm layer of chromatographic silica gel mixture.
Test solution 1—
Dissolve an accurately weighed quantity of Maleic Acid in acetone to obtain a solution having a concentration of about 100 mg per mL.
Test solution 2—
Transfer about 1.0 mL of Test solution 1 to a 50-mL volumetric flask, dilute with acetone to volume, and mix.
Maleic acid standard solution—
Dissolve an accurately weighed quantity of USP Maleic Acid RS in acetone to obtain a solution having a known concentration of about 2 mg per mL.
Fumaric acid standard solution—
Dissolve an accurately weighed quantity of USP Fumaric Acid RS in acetone to obtain a solution having a known concentration of about 1.5 mg per mL.
Resolution solution—
Prepare a mixture of Maleic acid standard solution and the Fumaric acid standard solution (1:1).
Developing solvent system—
Prepare a mixture of heptane, butanol, chloroform, and anhydrous formic acid (44:36:16:16).
Procedure—
Proceed as directed for Thin-Layer Chromatography under Chromatography  621
621 , using an unsaturated chamber. Separately apply 10 µL of the Resolution solution and 5 µL each of Test solution 1, Test solution 2, the Maleic acid standard solution, and the Fumaric acid standard solution. Dry the plate at 100
, using an unsaturated chamber. Separately apply 10 µL of the Resolution solution and 5 µL each of Test solution 1, Test solution 2, the Maleic acid standard solution, and the Fumaric acid standard solution. Dry the plate at 100 for 15 minutes, and examine the plate under short-wavelength UV light at 254 nm. The chromatogram obtained from the Resolution solution exhibits two clearly separated spots, and any spot corresponding to fumaric acid in the chromatogram of Test solution 1 does not exceed, in size or intensity, the principal spot obtained in the chromatogram of the Fumaric acid standard solution: not more than 1.5% of fumaric acid is found.
for 15 minutes, and examine the plate under short-wavelength UV light at 254 nm. The chromatogram obtained from the Resolution solution exhibits two clearly separated spots, and any spot corresponding to fumaric acid in the chromatogram of Test solution 1 does not exceed, in size or intensity, the principal spot obtained in the chromatogram of the Fumaric acid standard solution: not more than 1.5% of fumaric acid is found.
Limit of iron—
Diluted standard iron solution—
Immediately before use, dilute 1 volume of the Standard Iron Solution, prepared as directed under Iron  241
241 , with 9 volumes of water. [note—This solution contains the equivalent of 1 µg of iron per mL.]
, with 9 volumes of water. [note—This solution contains the equivalent of 1 µg of iron per mL.]
Potassium thiocyanate solution—
Dissolve 9.7 g of potassium thiocyanate in 100 mL of water.
Test solution—
Dissolve about 1 g of Maleic Acid, accurately weighed, in 10 mL of water. Add 2 mL of diluted hydrochloric acid and 0.05 mL of bromine TS. After 5 minutes, remove the excess of bromine with the aid of a current of air, add 3 mL of Potassium thiocyanate solution, and shake well.
Standard solution—
To 5 mL of Diluted standard iron solution add 6 mL of water. Add 1 mL of diluted hydrochloric acid and 0.05 mL of bromine TS. After 5 minutes, remove the excess of bromine with the aid of a current of air, add 3 mL of Potassium thiocyanate solution, and shake well.
Procedure—
Allow the Standard solution and the Test solution to stand for 5 minutes. Any red color in the Test solution is not more intense than that in the Standard solution: not more than 5 µg per g.
Assay—
Dissolve about 500 mg of Maleic Acid, accurately weighed, in 50 mL of water, and titrate with 1 N sodium hydroxide VS, using phenolphthalein TS as the indicator. Each mL of 1 N sodium hydroxide is equivalent to 58.04 mg of C4H4O4.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Robert H. Lafaver, B.A.
Scientist 1-301-816-8335 |
(EM105) Excipient Monographs 1 |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 1273
Pharmacopeial Forum: Volume No. 31(3) Page 815