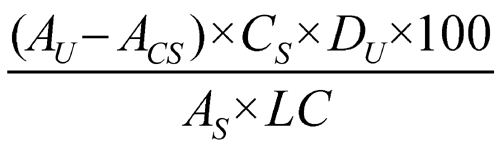Isotretinoin Capsules
» Isotretinoin Capsules contain not less than 90.0 percent and not more than 110.0 percent of the labeled amount of isotretinoin (C20H28O2).
Caution—Isotretinoin is teratogenic. Avoid inhalation and skin contact.
Packaging and storage—
Preserve in tight containers, protected from light. Store at controlled room temperature, in a dry place.
USP Reference standards  11
11 —
—
USP Isotretinoin RS.
USP Tretinoin RS.
USP Isotretinoin RS.
USP Tretinoin RS.
note—Avoid exposure to strong light, and use low-actinic glassware in the performance of the following procedures.
Identification—
The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
Dissolution  711
711 —
—
Medium—
stage 1:
simulated gastric fluid with pepsin, prepared freshly and purged with nitrogen.
stage 2:
0.13 N sodium hydroxide, prepared by transferring 5 g of sodium hydroxide to a 1-L volumetric flask and dissolving in and diluting with water to volume. Prepare fresh, and purge with nitrogen.
Apparatus
(see Disintegration  701
701 )—No disks; the apparatus is adjusted so that the bottom of the basket-rack assembly descends to 1.0 ± 0.1 cm from the inside bottom surface of the vessel on the downward stroke; the 10-mesh stainless steel cloth in the basket-rack assembly is replaced with a 40-mesh stainless steel cloth; a 10-mesh stainless-steel cloth is fitted to the top of the basket-rack assembly.
)—No disks; the apparatus is adjusted so that the bottom of the basket-rack assembly descends to 1.0 ± 0.1 cm from the inside bottom surface of the vessel on the downward stroke; the 10-mesh stainless steel cloth in the basket-rack assembly is replaced with a 40-mesh stainless steel cloth; a 10-mesh stainless-steel cloth is fitted to the top of the basket-rack assembly.
Time:
60 minutes.
Standard solution—
Transfer about 10 mg of USP Isotretinoin RS, accurately weighed, to a 200-mL low-actinic volumetric flask; add 25.0 mL of Stage 1 Medium and about 150 mL of Stage 2 Medium; sonicate until completely dissolved (about 20 minutes); and dilute with Stage 2 Medium to volume. Pass 20 mL of this solution through a suitable filter, discarding the first 5 mL. Dilute 5.0 mL of the filtrate with Stage 2 Medium to 50 mL.
Sample solution—
Perform a dissolution test on each of 6 Capsules: place 1 Capsule in one of the tubes in each of six basket-rack assemblies. Place each basket in a 1-L beaker containing 100 mL of Stage 1 Medium in a bath having a temperature of 37.0 ± 0.5 . Allow to stand for 30 minutes. Carefully add 800 mL of Stage 2 Medium to each beaker. With the disintegration apparatus operating, connect each basket-rack assembly to the drive rod in a timed sequence. After 60 minutes, withdraw 20 mL of Medium (Stage 1 and Stage 2), immediately pass the solution through a suitable 0.45-µm filter, discard the first 5 mL, and collect the solution in argon-charged, low-actinic glassware. Dilute, if necessary, using low-actinic glassware, with Stage 2 Medium, to obtain a theoretical concentration of about 0.0055 mg per mL of isotretinoin, assuming complete dissolution, based on the label claim.
. Allow to stand for 30 minutes. Carefully add 800 mL of Stage 2 Medium to each beaker. With the disintegration apparatus operating, connect each basket-rack assembly to the drive rod in a timed sequence. After 60 minutes, withdraw 20 mL of Medium (Stage 1 and Stage 2), immediately pass the solution through a suitable 0.45-µm filter, discard the first 5 mL, and collect the solution in argon-charged, low-actinic glassware. Dilute, if necessary, using low-actinic glassware, with Stage 2 Medium, to obtain a theoretical concentration of about 0.0055 mg per mL of isotretinoin, assuming complete dissolution, based on the label claim.
Capsule shell correction—
Empty the contents of 3 Capsules. Wash the Capsule shells in several 20-mL aliquots of chloroform. Allow the Capsule shells to air dry. Place the Capsule shells in a 1-L flask containing 100 mL of Stage 1 Medium and 800 mL of Stage 2 Medium. Allow the flask to stand for about 1 hour in a bath having a temperature of 37.0 ± 0.5 , stirring occasionally. Filter, and dilute as described for Sample solution.
, stirring occasionally. Filter, and dilute as described for Sample solution.
Procedure—
Determine the amount of C20H28O2 dissolved by employing UV absorption at the wavelength of maximum absorbance at about 343 nm, in portions of the Sample solution in comparison with the Standard solution, correcting for the Capsule shell absorbance, and using Medium (Stage 1 and Stage 2) as the blank. Calculate the percentage of C20H28O2 dissolved by the formula:
in which AU, ACS, and AS are the absorbances obtained from the Sample solution, the Capsule shell correction, and the Standard solution, respectively; CS is the concentration, in mg per mL, of the Standard solution; DU is the dilution factor of the Sample solution; 100 is the conversion factor to percentage; and LC is the Capsule label claim, in mg.
Tolerances—
Not less than 80% (Q) of the labeled amount of C20H28O2 is dissolved in 60 minutes.
Uniformity of dosage units  905
905 :
meet the requirements.
:
meet the requirements.
Chromatographic purity—
Methylene chloride reagent and Mobile phase—
Proceed as directed in the Assay.
Standard solution—
Dissolve an accurately weighed quantity of USP Tretinoin RS in Methylene chloride reagent to obtain a solution having a known concentration of about 0.5 mg per mL. Dilute an accurately measured volume of this solution quantitatively, and stepwise if necessary, with hexanes to obtain a solution having a known concentration of about 1 µg per mL.
Test solution—
Transfer 50.0 mL of the stock solution retained from the Assay preparation to a 200-mL volumetric flask, dilute with hexanes to volume, and mix to obtain a solution having a concentration of about 0.1 mg of isotretinoin per mL.
Chromatographic system—
Proceed as directed in the Assay, using the Standard preparation prepared in the Assay.
Procedure—
Separately inject equal volumes (about 50 µL) of the Standard solution and the Test solution into the chromatograph, and allow the Test solution to elute for not less than two times the retention time of isotretinoin. Record the chromatograms, and measure the peak responses: the peak response for any impurity is not more than that of the tretinoin response obtained from the Standard solution (1.0%); and the sum of all the peak responses, excluding that of isotretinoin, obtained from the Test solution, is not more than 1.5 times the tretinoin response obtained from the Standard solution (1.5%).
Assay—
Methylene chloride reagent—
Transfer 50 g of sodium bicarbonate to 1000 mL of methylene chloride, shake, and allow to stand overnight. At the time of use, filter suitable portions of this solution, and add 10 mg of butylated hydroxytoluene per mL.
Mobile phase—
Prepare a filtered and degassed mixture of hexanes, ethyl acetate, and glacial acetic acid (970:30:0.1). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard preparation—
Dissolve accurately weighed quantities of USP Isotretinoin RS and USP Tretinoin RS in Methylene chloride reagent to obtain a solution having known concentrations of about 1 mg of each Reference Standard per mL. Transfer 1.0 mL of this solution, and dilute quantitatively with hexanes to 100.0 mL to obtain a solution having known concentrations of about 0.01 mg of each Reference Standard per mL.
Assay preparation—
Weigh an accurately counted number of Capsules, equivalent to about 200 mg of isotretinoin, and calculate the average weight per Capsule. With a sharp blade, carefully open the Capsules, without loss of material, and transfer the contents by pipetting 5 mL of Methylene chloride reagent over each Capsule and rinsing with hexanes. Collect the washings in a 500-mL volumetric flask, dilute with hexanes to volume, and mix. [note—Reserve a portion of this stock solution for the Chromatographic purity test.] Transfer 5.0 mL of the stock solution to a 200-mL volumetric flask, dilute with hexanes to volume, and mix to obtain a solution having a concentration of 0.01 mg of isotretinoin per mL.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 365-nm detector and a 4.6-mm × 25-cm column containing packing L3. The flow rate is about 1 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative retention times for isotretinoin and tretinoin are about 0.75 and 1.00, respectively; the resolution, R, between isotretinoin and tretinoin is not less than 3.0; the tailing factor for the isotretinoin peak is not greater than 2.5; and the relative standard deviation for replicate injections is not more than 2.0%.
)—
The liquid chromatograph is equipped with a 365-nm detector and a 4.6-mm × 25-cm column containing packing L3. The flow rate is about 1 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative retention times for isotretinoin and tretinoin are about 0.75 and 1.00, respectively; the resolution, R, between isotretinoin and tretinoin is not less than 3.0; the tailing factor for the isotretinoin peak is not greater than 2.5; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 20 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of isotretinoin (C20H28O2) in each of the Capsules taken by the formula:
20,000(C/N)(rU / rS)
in which C is the concentration, in mg per mL, of USP Isotretinoin RS in the Standard preparation; N is the number of Capsules taken; and rU and rS are the isotretinoin peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Feiwen Mao, M.S.
Scientist 1-301-816-8320 |
(MDOOD05) Monograph Development-Ophthalmics Oncologics and Dermatologicals |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
|
| Margareth R.C. Marques, Ph.D.
Senior Scientist 1-301-816-8106 |
(BPC05) Biopharmaceutics05 |
USP32–NF27 Page 2723
Pharmacopeial Forum: Volume No. 34(4) Page 942
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.