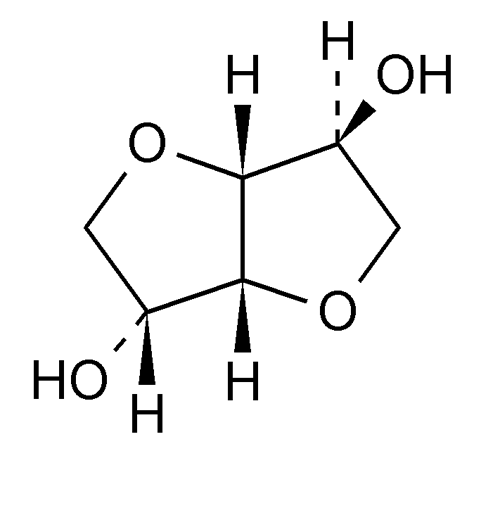
Isosorbide Concentrate
» Isosorbide Concentrate is an aqueous solution containing, in each 100 g, not less than 70.0 g and not more than 80.0 g of C6H10O4.
Packaging and storage—
Preserve in tight, light-resistant containers.
Labeling—
The label states that this article is not intended for direct administration to humans or animals.
Identification—
[note—Isosorbide is hygroscopic. Take precautions to protect isolated isosorbide crystals from atmospheric moisture.]
A:
Dry a portion of it in an evaporating dish over phosphorus pentoxide at 70 and at a pressure of 50 mm of mercury for 48 hours, changing the phosphorus pentoxide after 24 hours. Scratch the bottom of the dish with a glass rod or seed with a crystal of isosorbide, if necessary, to initiate crystallization: the crystals so obtained melt between 60
and at a pressure of 50 mm of mercury for 48 hours, changing the phosphorus pentoxide after 24 hours. Scratch the bottom of the dish with a glass rod or seed with a crystal of isosorbide, if necessary, to initiate crystallization: the crystals so obtained melt between 60 and 63
and 63 when tested by the procedure for Class I substances (see Melting Range or Temperature
when tested by the procedure for Class I substances (see Melting Range or Temperature  741
741 ).
).
B:
The IR absorption spectrum of a potassium bromide dispersion of the crystals obtained as directed in Identification test A exhibits maxima and minima only at the same wavelengths as that of a similar preparation of USP Isosorbide RS.
Specific rotation  781S
781S :
between +44.5
:
between +44.5 and +47.0
and +47.0 .
.
Test solution:
80 mg of isosorbide per mL, in water.
Water, Method I  921
921 :
between 24.0% and 26.0%.
:
between 24.0% and 26.0%.
Residue on ignition  281
281 :
not more than 0.01%.
:
not more than 0.01%.
Heavy metals, Method II  231
231 :
not more than 5 ppm, calculated on the anhydrous basis.
:
not more than 5 ppm, calculated on the anhydrous basis.
Periodate consumption—
Dilute about 15 g, accurately weighed, with 25 mL of water, and add 50.0 mL of a solution prepared by dissolving 5.4 g of periodic acid in 100 mL of water and adding 1900 mL of glacial acetic acid. Allow to stand for 1 hour. Add 20 mL of potassium iodide TS, and titrate with 0.1 N sodium thiosulfate VS to the disappearance of the brown color. Add 3 mL of starch TS, and complete the titration. Perform a blank determination, and note the difference in volumes required. If the volume required for the specimen is less than 0.8 of that required for the blank, repeat the procedure with a smaller specimen. The difference in volume corresponds to not more than 0.20 mL of 0.1 N sodium thiosulfate for each g of Concentrate taken.
Acid value—
Dilute about 15 g, accurately weighed, with 50 mL of water, and titrate with 0.02 N potassium hydroxide VS to a phenolphthalein endpoint. Perform a blank determination, and make any necessary correction. Calculate the acid value taken by the formula:
56.11(AN / W)
in which A is the number of mL of potassium hydroxide VS consumed; N is its normality; and W is the weight, in g, of Concentrate taken. The limit is 0.5, calculated on the anhydrous basis.
Methyl ethyl ketone—
Internal standard solution—
Prepare a solution in water containing about 1 mg per mL of methyl isobutyl ketone.
Standard preparation—
Prepare a solution in water containing an accurately known concentration of methyl ethyl ketone equivalent to about 1 mg per mL. Pipet 5 mL of this solution into a 100-mL volumetric flask, add 5.0 mL of Internal standard solution, add water to volume, and mix.
Test preparation—
Pipet 5 mL of Internal standard solution into a 100-mL volumetric flask, add Concentrate to volume, and mix.
Support—
Place about 90 g of unsilanized support S1A in a crystallizing dish, and cover it with chloroform. Stir the mixture thoroughly, and carefully remove the supernatant chloroform with an aspirator. Spread the moist support on a clean surface, and allow it to air-dry. Place the dried support in the crystallizing dish, and cover it with 0.5 N alcoholic potassium hydroxide TS. Allow it to stand for one-half hour with occasional stirring. Carefully pour off the supernatant alcoholic potassium hydroxide solution, and wash the moist support with water until the washing is neutral to phenolphthalein indicator. Spread the moist support on a clean surface, and allow it to air-dry.
Chromatographic system—
The gas chromatograph is equipped with a flame-ionization detector and a 0.6-m × 2-mm column packed with 25% liquid phase G16 on unsilanized acid- and base-washed Support which has been washed with chloroform, and conditioned as directed (see Chromatography  621
621 ). The column is maintained at 70
). The column is maintained at 70 , and nitrogen is used as the carrier gas at a flow rate of about 30 mL per minute. In a suitable chromatogram, the relative standard deviation of five replicate injections is not more than 3.0%, and the resolution is not less than 2.0.
, and nitrogen is used as the carrier gas at a flow rate of about 30 mL per minute. In a suitable chromatogram, the relative standard deviation of five replicate injections is not more than 3.0%, and the resolution is not less than 2.0.
Procedure—
Inject about 3 µL of the Standard preparation into the gas chromatograph, record the chromatogram, and measure the peak response of each component. [note—Clean the syringe after each injection with pentane. Do not use acetone.] Similarly inject about 3 µL of the Test preparation, record the chromatogram, and measure the peak response of each component. Calculate the quantity, in mg, of methyl ethyl ketone in each mL of the Concentrate taken by the formula:
(1 / 0.95)C(RU / RS)
in which C is the concentration, in mg per mL, of methyl ethyl ketone in the Standard preparation, and RU and RS are the ratios of the response of the methyl ethyl ketone to the response of the internal standard obtained from the Test preparation and the Standard preparation, respectively. The limit is 0.05 mg per mL.
Assay—
Internal standard solution—
Dissolve a suitable quantity of triethylene glycol in water to obtain a solution containing about 15 mg per mL.
Standard solution—
Prepare a solution of USP Isosorbide RS in water containing an accurately known concentration equivalent to about 25 mg of C6H10O4 per mL.
Standard preparations—
Pipet 2-, 3-, 4-, and 5-mL quantities of Standard solution into separate 50-mL volumetric flasks, add 5.0 mL of Internal standard solution to each, add water to volume, and mix.
Assay preparation—
Transfer about 200 mg of Concentrate, accurately weighed, to a 100-mL volumetric flask, add 10.0 mL of Internal standard solution, add water to volume, and mix.
Chromatographic system—
The gas chromatograph is equipped with a flame-ionization detector and a 3-mm × 0.6-m glass column packed with support S9. The column is maintained at 230 , and nitrogen is used as the carrier gas. The retention time of the isosorbide peak is about 1.5, relative to that of triethylene glycol.
, and nitrogen is used as the carrier gas. The retention time of the isosorbide peak is about 1.5, relative to that of triethylene glycol.
System suitability and standard curve—
Inject 1-µL portions of each Standard preparation, and record each peak response. Plot the ratio of the peak response of isosorbide to that of triethylene glycol versus the concentration, in mg per mL, of isosorbide in the respective Standard preparation. The analytical system is suitable for conducting the assay if the correlation coefficient for the Standard curve is greater than 0.980, the resolution, R, is not less than 1.5, and neither tailing factor exceeds 2.0.
Procedure—
Inject a 1-µL portion of the Assay preparation, record the peak responses for the two major peaks, calculate the ratio of the peak responses, and determine the concentration, C, in mg per mL, of isosorbide in the Assay preparation by reference to the Standard curve. Calculate the quantity, in mg, of C6H10O4 in the Concentrate taken by the formula:
100C.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Sujatha Ramakrishna, Ph.D.
Scientist 1-301-816-8349 |
(MDCV05) Monograph Development-Cardiovascular |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 2711
Pharmacopeial Forum: Volume No. 28(3) Page 766
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.