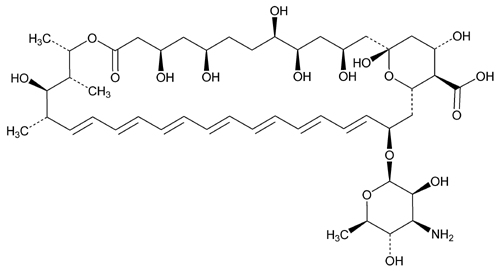
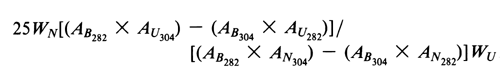Amphotericin B
Amphotericin B.
Amphotericin B.
[1R-(1R*,3S*,5R*,6R*,9R*,11R*,15S*,16R*,17R*,18S*,19E,21E,23E,25E,27E,29E,31E,33R*,35S*,36R*,37S*)]-33- [(3-Amino-3,6-dideoxy-
» Amphotericin B has a potency of not less than 750 µg of C47H73NO17 per mg, calculated on the dried basis.
Packaging and storage—
Preserve in tight, light-resistant containers, and store in a cold place.
Labeling—
Label it to state whether it is intended for use in preparing dermatological and oral dosage forms or parenteral dosage forms.
Identification, Ultraviolet Absorption  197U
197U —
—
Spectral range 1:
240 to 320 nm.
Solution 1:
prepared as directed for Test preparation in the Limit of amphotericin A, and compare its absorbance to that of the Amphotericin B standard preparation. An extra peak may occur at 304 nm in the spectrum of this solution.
Spectral range 2:
320 to 400 nm.
Solution 2:
prepared as directed for Test preparation in the Limit of amphotericin A and then diluted with 9 volumes of methanol. Compare its absorbance to that of a similar dilution of the Amphotericin B standard preparation.
Loss on drying  731
731 —
Dry about 100 mg, accurately weighed, in a capillary-stoppered bottle in vacuum at a pressure not exceeding 5 mm of mercury at 60
—
Dry about 100 mg, accurately weighed, in a capillary-stoppered bottle in vacuum at a pressure not exceeding 5 mm of mercury at 60 for 3 hours: it loses not more than 5.0% of its weight.
for 3 hours: it loses not more than 5.0% of its weight.
Residue on ignition  281
281 :
not more than 0.5%, the charred residue being moistened with 2 mL of nitric acid and 5 drops of sulfuric acid. [note—Amphotericin B intended for use in preparing dermatological creams, lotions, and ointments, and oral suspensions and capsules, yields not more than 3.0%.]
:
not more than 0.5%, the charred residue being moistened with 2 mL of nitric acid and 5 drops of sulfuric acid. [note—Amphotericin B intended for use in preparing dermatological creams, lotions, and ointments, and oral suspensions and capsules, yields not more than 3.0%.]
Limit of amphotericin A—
Test preparation—
Dissolve about 50 mg of Amphotericin B, accurately weighed, in 10.0 mL of dimethyl sulfoxide in a 50-mL volumetric flask, dilute with methanol to volume, and mix. Transfer 4.0 mL of this solution to a 50-mL volumetric flask, dilute with methanol to volume, and mix.
Nystatin standard preparation—
Dissolve about 20 mg of USP Nystatin RS, accurately weighed, in 40.0 mL of dimethyl sulfoxide in a 200-mL volumetric flask, dilute with methanol to volume, and mix. Transfer 4.0 mL of this solution to a 50-mL volumetric flask, dilute with methanol to volume, and mix.
Amphotericin B standard preparation—
Dissolve about 50 mg of USP Amphotericin B RS, accurately weighed, in 10.0 mL of dimethyl sulfoxide in a 50-mL volumetric flask, dilute with methanol to volume, and mix. Transfer 4.0 mL of this solution to a 50-mL volumetric flask, dilute with methanol to volume, and mix. Prepare this solution fresh daily.
Procedure—
Concomitantly determine the absorbances of the Nystatin and Amphotericin B standard preparations and the Test preparation in 1-cm cells at 304 nm and at 282 nm, with a suitable spectrophotometer, using a 1 in 62.5 solution of dimethyl sulfoxide in methanol as the blank. Calculate the percentage of amphotericin A taken by the formula:
in which WN is the weight, in mg, of USP Nystatin RS taken, AB282 and AB304 are the absorbances of the Amphotericin B standard preparation at 282 nm and 304 nm, respectively, AN282 and AN304 are the absorbances of the Nystatin standard preparation at 282 nm and 304 nm, respectively, AU282 and AU304 are the absorbances of the Test preparation at 282 nm and 304 nm, respectively, and WU is the weight, in mg, of the Amphotericin B taken: not more than 5%, calculated on the dried basis, is found. [note—Amphotericin B intended for use in preparing dermatological creams, lotions, and ointments, and oral suspensions and capsules, contains not more than 15% of amphotericin A, calculated on the dried basis.]
Assay—
Proceed with amphotericin B as directed under Antibiotics—Microbial Assays  81
81 .
.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Ahalya Wise, M.S.
Scientist 1-301-816-8161 |
(MDANT05) Monograph Development-Antibiotics |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 1547