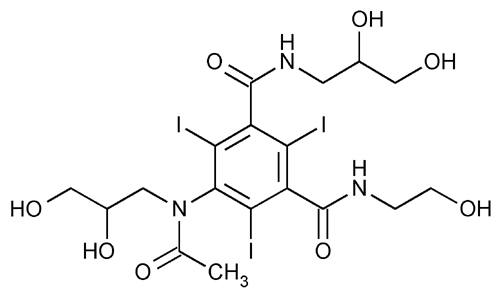
Ioxilan
» Ioxilan contains not less than 98.0 percent and not more than 102.0 percent of C18H24I3N3O8, calculated on the anhydrous and methanol-free basis.
Packaging and storage—
Preserve in well-closed, light-resistant containers. Store at 25 , excursions permitted between 15
, excursions permitted between 15 and 30
and 30 .
.
Identification—
Bacterial endotoxins  85
85 —
It contains not more than 0.2 USP Endotoxin Unit per 50 mg of iodine.
—
It contains not more than 0.2 USP Endotoxin Unit per 50 mg of iodine.
Heavy metals, Method I  231
231 :
0.002%.
:
0.002%.
pH  791
791 :
between 5.0 and 7.5, in a solution (1 in 10). [note—Heat gently, if necessary, to dissolve. Cool to room temperature before proceeding.]
:
between 5.0 and 7.5, in a solution (1 in 10). [note—Heat gently, if necessary, to dissolve. Cool to room temperature before proceeding.]
Water, Method I  921
921 :
not more than 4.0%.
:
not more than 4.0%.
Free iodine—
Transfer 2 g to a stoppered flask, add 12 mL of water, and swirl to dissolve. [note—Heat gently, if necessary, to dissolve. Cool to room temperature before proceeding.] Add 2.5 mL of 2 N sulfuric acid and 4 mL of toluene, stopper the flask, and shake for 1 minute. Allow the layers to separate: the toluene layer shows no red color. Reserve the contents of the flask for use as the test solution in the test for Free iodide.
Free iodide—
Prepare a solution of potassium iodide in water containing 1000 µg of iodide per mL. Quantitatively dilute portions of this solution with water to obtain standard solutions having concentrations of 100, 50, 25, and 10 µg of iodide per mL. Transfer 2 mL of each standard solution to separate stoppered flasks, and add 12 mL of water to each flask. Add 14 mL of water to an additional flask to serve as the blank. Add 2.5 mL of 2 N sulfuric acid and 4 mL of toluene to the flasks containing the standard solutions and the blank, stopper the flasks, and shake for 1 minute. To the test solution obtained in the test for Free iodine, each of the standard solutions, and the blank, add 0.5 mL of sodium nitrite solution (2 in 100), stopper the flasks, and shake vigorously for 1 minute. Allow the layers to separate, transfer the toluene layers to separate centrifuge tubes, and centrifuge for 1 minute. Concomitantly determine the absorbances of the test solution and the standard solutions against the blank. From a linear regression equation calculated from the concentrations and absorbances of the iodide standard solutions, determine the content of iodide in the Ioxilan: the limit is 30 µg of iodide per g of Ioxilan.
Free aromatic amine—
[note—Protect the Standard solution, the test solution, and the blank from light throughout the test.]
pH 10 Buffer—
To 33.7 g of ammonium chloride add 285 mL of ammonium hydroxide, dilute with water to 500 mL, and mix.
Procedure—
Transfer 20 mg of USP Ioxilan Related Compound A RS, accurately weighed, to a 200-mL volumetric flask, dissolve in a small volume of hot water, cool, dilute with water to volume, and mix. Transfer 2.5 mL of this solution to a 50-mL volumetric flask, add 12 mL of water, and mix. Transfer 0.5 g of Ioxilan to a second 50-mL volumetric flask, add 12 mL of water, and shake to dissolve. [note—Heat gently, if necessary, to dissolve. Cool to room temperature before proceeding.] To a third 50-mL volumetric flask, add 12 mL of water to serve as the blank, and place the flasks containing the Standard solution, the test solution, and the blank in an ice bath. [note—In conducting the following steps, keep the flasks in the ice bath as much as possible until all of the reagents have been added.] Treat each of the flasks as follows. Add 5 mL of sodium nitrite solution (0.5 in 100), and shake. [Caution—Considerable pressure is produced with the addition of each reagent.
]
Add 10 mL of 1 N hydrochloric acid, swirl, and allow to stand for 2 minutes. Add 3 drops of a 1 in 10 solution of  -naphthol in alcohol, swirl, and allow to stand for 2 minutes. Add 3.5 mL of pH 10 Buffer, swirl, and allow to stand out of the ice bath for 2 minutes. Degas all solutions in a water bath at 25
-naphthol in alcohol, swirl, and allow to stand for 2 minutes. Add 3.5 mL of pH 10 Buffer, swirl, and allow to stand out of the ice bath for 2 minutes. Degas all solutions in a water bath at 25 for 10 minutes, dilute with water to 50 mL, and mix. Within 15 minutes of this final dilution, concomitantly determine the absorbances of the test solution and the Standard solution at the wavelength of maximum absorbance at about 485 nm, with a suitable spectrophotometer, against the blank. The absorbance of the test solution is not greater than that of the Standard solution (0.05%).
for 10 minutes, dilute with water to 50 mL, and mix. Within 15 minutes of this final dilution, concomitantly determine the absorbances of the test solution and the Standard solution at the wavelength of maximum absorbance at about 485 nm, with a suitable spectrophotometer, against the blank. The absorbance of the test solution is not greater than that of the Standard solution (0.05%).
Residual methanol—
Standard stock solution—
Transfer 200 mg of methanol to a tared 200-mL volumetric flask containing 20 mL of water, dilute with water to volume, and mix. Transfer 1.0 mL of this solution to a 100-mL volumetric flask containing 20 mL of water, dilute with water to volume, and mix.
Internal standard solution—
Transfer 200 mg of dehydrated alcohol to a tared 200-mL volumetric flask containing 20 mL of water, dilute with water to volume, and mix. Transfer 10.0 mL of this solution to a 100-mL volumetric flask, dilute with water to volume, and mix.
Standard solutions A, B, C, and D—
Transfer 0.5, 1.0, 2.0, and 4.0 mL of Standard stock solution to separate 10-mL volumetric flasks, each containing 2 mL of water. Add 1.0 mL of Internal standard solution to each flask, dilute with water to volume, and mix to obtain Standard solutions A, B, C, and D having concentrations of 0.5, 1.0, 2.0, and 4.0 mg of methanol per L, respectively.
Test solution—
Transfer about 1 g of Ioxilan to a tared 10-mL ampul, and add water to the ampul to a final weight of 10.45 g, taking care to avoid leaving water in the neck of the ampul. Seal the ampul, and immerse it in a water bath at 90 until the Ioxilan is dissolved. Mix, and allow to cool to room temperature. Mix again, open the ampul, and dilute the contents with a volume of water suitable to obtain a methanol concentration within the range of the standard curve (between 0.5 and 4 mg per L), allowing for the addition of 1.0 mL of Internal standard solution.
until the Ioxilan is dissolved. Mix, and allow to cool to room temperature. Mix again, open the ampul, and dilute the contents with a volume of water suitable to obtain a methanol concentration within the range of the standard curve (between 0.5 and 4 mg per L), allowing for the addition of 1.0 mL of Internal standard solution.
Chromatographic system (see System Suitability under Chromatography  621
621 )—
The gas chromatograph is equipped with a flame-ionization detector and a 10-m × 0.53-mm fused silica capillary column coated with support S2. The column temperature is programmed to increase from 45
)—
The gas chromatograph is equipped with a flame-ionization detector and a 10-m × 0.53-mm fused silica capillary column coated with support S2. The column temperature is programmed to increase from 45 to 80
to 80 at a rate of 5
at a rate of 5 per minute. The injection port temperature is maintained at 180
per minute. The injection port temperature is maintained at 180 , and the detector temperature is maintained at 200
, and the detector temperature is maintained at 200 . Helium is used as the carrier gas at a flow rate of about 30 mL per minute. Chromatograph Standard solution D, and record the peak responses as directed for Procedure: the resolution, R, between the methanol and alcohol peaks is not less than 10; the tailing factor for the methanol peak is not more than 3.0; and the relative standard deviation for replicate injections is not more than 5.0%.
. Helium is used as the carrier gas at a flow rate of about 30 mL per minute. Chromatograph Standard solution D, and record the peak responses as directed for Procedure: the resolution, R, between the methanol and alcohol peaks is not less than 10; the tailing factor for the methanol peak is not more than 3.0; and the relative standard deviation for replicate injections is not more than 5.0%.
Procedure—
Separately inject equal volumes (about 1 µL) of each Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the areas of the peak responses. From a linear regression equation calculated from the ratios of the peak responses of methanol to those of alcohol for each of the Standard solutions versus the methanol concentrations, in mg per L, determine the methanol content in the Test solution: not more than 2.0% is found.
Limit of serinol impurity—
Mobile phase—
Prepare a filtered and degassed mixture of acetonitrile and water (91:9). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard solution—
Transfer about 60 mg of USP Ioxilan RS to a 10-mL volumetric flask, add 1 mL of water, and heat at not more than 80 to dissolve. Cool to room temperature, dilute with acetonitrile to volume, and mix.
to dissolve. Cool to room temperature, dilute with acetonitrile to volume, and mix.
Test solution—
Using about 60 mg of Ioxilan, proceed as directed for Standard solution.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 245-nm detector and a 25-cm × 4.6-mm stainless steel column that contains 5-µm packing L18. The flow rate is about 2 mL per minute, and the column temperature is maintained at 30
)—
The liquid chromatograph is equipped with a 245-nm detector and a 25-cm × 4.6-mm stainless steel column that contains 5-µm packing L18. The flow rate is about 2 mL per minute, and the column temperature is maintained at 30 . Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the retention time of the serinol peak is about 0.9 relative to that of the larger of the ioxilan peaks; the resolution, R, between the larger of the ioxilan peaks and the serinol peak is not less than 1.0; and the relative standard deviation of the response of the serinol peak for replicate injections is not more than 2.0%.
. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the retention time of the serinol peak is about 0.9 relative to that of the larger of the ioxilan peaks; the resolution, R, between the larger of the ioxilan peaks and the serinol peak is not less than 1.0; and the relative standard deviation of the response of the serinol peak for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 10 µL) of the Test solution and the Standard solution into the chromatograph, and allow the chromatogram to run for about 35 minutes. Measure the areas of the peak responses, and determine the percentage of serinol in the portion of Ioxilan taken: not more than 0.5% is found.
Chromatographic purity—
Mobile phase—
Prepare a filtered and degassed mixture of acetonitrile and water (87:13). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard solution—
Transfer about 60 mg of USP Ioxilan RS to a 10-mL volumetric flask, and add 1 mL of water. [note—Heat gently, if necessary, to dissolve. Cool to room temperature before proceeding.] Dilute with acetonitrile to volume, and mix.
Test solution—
Using about 60 mg of Ioxilan, proceed as directed for Standard solution.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 245-nm detector and a 25-cm × 4.6-mm stainless steel column that contains 5-µm packing L8. The column is maintained at 30
)—
The liquid chromatograph is equipped with a 245-nm detector and a 25-cm × 4.6-mm stainless steel column that contains 5-µm packing L8. The column is maintained at 30 , and the flow rate is about 1 mL per minute. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the resolution, R, between the two largest impurity peaks eluting immediately after the second ioxilan isomer peak is not less than 0.3.
, and the flow rate is about 1 mL per minute. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the resolution, R, between the two largest impurity peaks eluting immediately after the second ioxilan isomer peak is not less than 0.3.
Procedure—
Separately inject equal volumes (about 10 µL) of the Test solution and the Standard solution into the chromatograph, record the chromatograms, and measure the areas of the peak responses. Determine the percentage of each impurity (excluding the serinol impurity, which has a retention time of 0.9 relative to the larger ioxilan isomer) in the portion of Ioxilan taken: not more than 0.5% of any individual impurity is found; and the total of all impurities is not more than 1.5%.
Assay—
Transfer about 500 mg of Ioxilan, accurately weighed, to a 100-mL round-bottom flask. Add 1 g of powdered zinc and 40 mL of 1.25 N sodium hydroxide, connect the flask to a reflux condenser that is cooled with water at 5 to 10
to 10 , add a few glass boiling beads, and gently reflux the mixture for 1 hour. Allow the flask to cool to room temperature, and rinse the condenser with 10 to 20 mL of water. Disconnect the flask from the condenser, add 5 mL of glacial acetic acid, mix, and allow the mixture to cool. Pass through general purpose fast-flowing filter paper mixture. Rinse the flask and the filter with 1 N acetic acid, and add the rinsings to the filtrate. Add 5 mL of tetrabromophenolphthalein ethyl ester TS, and titrate with 0.1 N silver nitrate VS, with continuous stirring, until the precipitate turns green. Each mL of 0.1 N silver nitrate is equivalent to 26.37 mg of C18H24I3N3O8.
, add a few glass boiling beads, and gently reflux the mixture for 1 hour. Allow the flask to cool to room temperature, and rinse the condenser with 10 to 20 mL of water. Disconnect the flask from the condenser, add 5 mL of glacial acetic acid, mix, and allow the mixture to cool. Pass through general purpose fast-flowing filter paper mixture. Rinse the flask and the filter with 1 N acetic acid, and add the rinsings to the filtrate. Add 5 mL of tetrabromophenolphthalein ethyl ester TS, and titrate with 0.1 N silver nitrate VS, with continuous stirring, until the precipitate turns green. Each mL of 0.1 N silver nitrate is equivalent to 26.37 mg of C18H24I3N3O8.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Ian DeVeau, Ph.D.
Director, Veterinary Drugs and Radiopharmaceuticals 1-301-816-8178 |
(RMI05) Radiopharmaceuticals and Medical Imaging Agents 05 |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
|
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance |
USP32–NF27 Page 2680
Pharmacopeial Forum: Volume No. 29(6) Page 1911