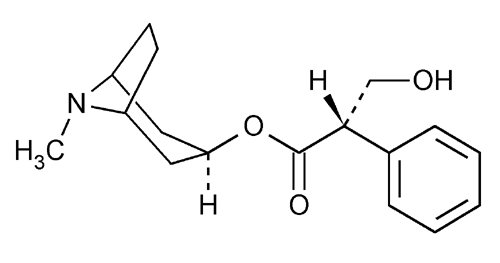
Hyoscyamine
Benzeneacetic acid,
1
» Hyoscyamine contains not less than 98.0 percent and not more than 101.0 percent of C17H23NO3, calculated on the dried basis.
Packaging and storage—
Preserve in tight, light-resistant containers.
Identification—
A:
Transfer 30 mg of Hyoscyamine and 36 mg of USP Hyoscyamine Sulfate RS to individual 60-mL separators with the aid of 5-mL portions of water. To each separator add 1.5 mL of 1 N sodium hydroxide and 10 mL of chloroform. Shake for 1 minute, allow the layers to separate, and filter the chloroform extracts through separate filters of about 2 g of anhydrous granular sodium sulfate supported on pledgets of glass wool. Extract each aqueous layer with two additional 10-mL portions of chloroform, filtering and combining with the respective main extracts. Evaporate the chloroform solutions under reduced pressure to dryness, and dissolve each residue in 10 mL of carbon disulfide: the IR absorption spectrum, determined in a 1-mm cell, of the solution obtained from the test specimen exhibits maxima only at the same wavelengths as that of the solution obtained from the Reference Standard.
B:
Dissolve 60 mg in 1 mL of 0.2 N hydrochloric acid, and add gold chloride TS, dropwise with shaking, until a definite precipitate separates. Add a small amount of 3 N hydrochloric acid, dissolve the precipitate with the aid of heat, and then allow to cool: lustrous golden yellow scales are formed (distinction from atropine and scopolamine).
Loss on drying  731
731 —
Dry it in vacuum over silica gel to constant weight: it loses not more than 0.2% of its weight.
—
Dry it in vacuum over silica gel to constant weight: it loses not more than 0.2% of its weight.
Residue on ignition  281
281 :
not more than 0.1%.
:
not more than 0.1%.
Limit of foreign alkaloids and other impurities—
Prepare a solution of it in methanol containing 20 mg per mL, and by quantitative dilution of a portion of this solution with methanol, prepare a second solution of Hyoscyamine containing 1 mg per mL. Apply 25 µL of the first (20 mg per mL) Hyoscyamine solution, 1 µL of the second (1 mg per mL) Hyoscyamine solution, and 5 µL of a methanol solution of USP Hyoscyamine Sulfate RS containing 24 mg per mL to a suitable thin-layer chromatographic plate (see Chromatography  621
621 ) coated with a 0.5-mm layer of chromatographic silica gel. Allow the spots to dry, and develop the chromatogram in a solvent system consisting of a mixture of chloroform, acetone, and diethylamine (5:4:1) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, and allow the solvent to evaporate. Locate the spots on the plate by spraying with potassium iodoplatinate TS. The RF value of the principal spot obtained from each test solution corresponds to that obtained from the Standard solution, and no secondary spot obtained from the first Hyoscyamine solution exhibits intensity equal to or greater than the principal spot obtained from the second Hyoscyamine solution (0.2%).
) coated with a 0.5-mm layer of chromatographic silica gel. Allow the spots to dry, and develop the chromatogram in a solvent system consisting of a mixture of chloroform, acetone, and diethylamine (5:4:1) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, and allow the solvent to evaporate. Locate the spots on the plate by spraying with potassium iodoplatinate TS. The RF value of the principal spot obtained from each test solution corresponds to that obtained from the Standard solution, and no secondary spot obtained from the first Hyoscyamine solution exhibits intensity equal to or greater than the principal spot obtained from the second Hyoscyamine solution (0.2%).
Assay—
Dissolve about 500 mg of Hyoscyamine, accurately weighed, in 50 mL of glacial acetic acid, add 1 drop of crystal violet TS, and titrate with 0.1 N perchloric acid VS to a green endpoint. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 28.94 mg of C17H23NO3.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Elena Gonikberg, Ph.D.
Senior Scientist 1-301-816-8251 |
(MDGRE05) Monograph Development-Gastrointestinal Renal and Endocrine |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 2600