Heparin Calcium
Change to read:
» Heparin Calcium is the calcium salt of sulfated glycosaminoglycans present as a mixture of heterogeneous molecules of mixed mucopolysaccharide nature varying in molecular weights. It is present in mammalian tissues and is usually obtained from the intestinal mucosa or other suitable tissues of domestic mammals used for food by humans.  The sourcing of heparin material must be specified in compliance with applicable regulatory requirements. The manufacturing process must be validated to demonstrate clearance and inactivation of relevant infectious and adventitious agents (e.g., viruses, TSE agents). See Viral Safety Evaluation of Biotechnology Products Derived From Cell Lines of Human or Animal Origin
The sourcing of heparin material must be specified in compliance with applicable regulatory requirements. The manufacturing process must be validated to demonstrate clearance and inactivation of relevant infectious and adventitious agents (e.g., viruses, TSE agents). See Viral Safety Evaluation of Biotechnology Products Derived From Cell Lines of Human or Animal Origin  1050
1050 for general guidance on viral safety evaluation.
for general guidance on viral safety evaluation. (RB 18-Jun-2008) It is purified to retain a combination of activities against different fractions of the blood clotting sequence. It is composed of polymers of alternating derivatives of
(RB 18-Jun-2008) It is purified to retain a combination of activities against different fractions of the blood clotting sequence. It is composed of polymers of alternating derivatives of 
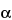 -
- (RB 18-Jun-2008) d-glucosamine (N-sulfated, O-sulfated, or N-acetylated) and uronic acid (
(RB 18-Jun-2008) d-glucosamine (N-sulfated, O-sulfated, or N-acetylated) and uronic acid (
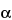 -
- (RB 18-Jun-2008) l-iduronic acid or
(RB 18-Jun-2008) l-iduronic acid or 
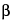 -
- (RB 18-Jun-2008) d-glucuronic acid) joined by glycosidic linkages. The component activities of the mixture are in ratios corresponding to those shown by the USP Heparin Sodium Reference Standard. Some of these components have the property of prolonging the clotting time of blood. This occurs through the formation of a complex of each component with the plasma proteins antithrombin III and heparin cofactor II to potentiate the inactivation of thrombin. Other coagulation proteases in the clotting sequence, such as activated factor X (factor Xa), are also inhibited. The potency of Heparin Calcium, calculated on the dried basis, is not less than 140 USP Heparin Units in each mg, and not less than 90.0 percent and not more than 110.0 percent of the potency stated on the label. Heparin Calcium is essentially free from sodium.
(RB 18-Jun-2008) d-glucuronic acid) joined by glycosidic linkages. The component activities of the mixture are in ratios corresponding to those shown by the USP Heparin Sodium Reference Standard. Some of these components have the property of prolonging the clotting time of blood. This occurs through the formation of a complex of each component with the plasma proteins antithrombin III and heparin cofactor II to potentiate the inactivation of thrombin. Other coagulation proteases in the clotting sequence, such as activated factor X (factor Xa), are also inhibited. The potency of Heparin Calcium, calculated on the dried basis, is not less than 140 USP Heparin Units in each mg, and not less than 90.0 percent and not more than 110.0 percent of the potency stated on the label. Heparin Calcium is essentially free from sodium.
note—The USP Heparin Unit is defined by the USP Heparin Sodium Reference Standard, independent of International Units. The respective units are not equivalent (see General Notices). Unit for Anti-factor Xa activity is defined by the USP Heparin Sodium Reference Standard.
Packaging and storage—
Preserve in tight containers, and store at a temperature below 40 , preferably at room temperature.
, preferably at room temperature.
Labeling—
Label it to indicate the tissue and the animal species from which it is derived.
Change to read:
USP Reference standards USP Endotoxin RS.
USP Heparin Sodium RS.
USP Heparin Sodium System Suitability RS
 .
.
USP Heparin Sodium Identification RS.
Change to read:
Identification—
B:
1H NMR spectrum (see Nuclear Magnetic Resonance  761
761 )—Proceed as directed in Identification test B under Heparin Sodium, substituting Heparin Calcium for Heparin Sodium.
)—Proceed as directed in Identification test B under Heparin Sodium, substituting Heparin Calcium for Heparin Sodium.
Bacterial endotoxins  85
85 —
It contains not more than 0.03 Endotoxin Unit per USP Heparin Unit.
—
It contains not more than 0.03 Endotoxin Unit per USP Heparin Unit.
Sterility  71
71 (where it is labeled as sterile)—
It meets the requirements.
(where it is labeled as sterile)—
It meets the requirements.
pH  791
791 :
between 5.0 and 7.5, in a solution (1 in 100).
:
between 5.0 and 7.5, in a solution (1 in 100).
Loss on drying  731
731 —
Dry it in vacuum at 60
—
Dry it in vacuum at 60 for 3 hours: it loses not more than 5.0% of its weight.
for 3 hours: it loses not more than 5.0% of its weight.
Residue on ignition  281
281 :
between 28.0% and 41.0%.
:
between 28.0% and 41.0%.
Protein—
To 1 mL of a solution (1 in 100) add 5 drops of trichloroacetic acid solution (1 in 5): no precipitate or turbidity forms.
Heavy metals, Method II  231
231 :
0.003%.
:
0.003%.
Anti-factor Xa activity—
Proceed as directed in the test for Anti-factor Xa activity under Heparin Sodium, except to use Heparin Calcium instead of Heparin Sodium to prepare the Test solutions. The specified results are obtained.
Nitrogen content, Method I  461
461 :
between 1.3% and 2.5%, calculated on the dried basis, the procedure for Nitrates and Nitrites Absent being used.
:
between 1.3% and 2.5%, calculated on the dried basis, the procedure for Nitrates and Nitrites Absent being used.
Assay—
Proceed as directed in the Assay under Heparin Sodium, except to use Heparin Calcium solution to prepare the Assay preparation. In the equation for R in the Calculation section, vU is the amount in mg of Heparin Calcium per mL of the Assay preparation. The potency of Heparin Calcium in USP Heparin Units per mg is P* = antilog M.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Anita Y. Szajek, Ph.D.
Senior Scientist 1-301-816-8325 |
(BBBBP05) Biologics and Biotechnology - Blood and Blood Products |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
|
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance | |
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance |
USP32–NF27 Page 2551
Pharmacopeial Forum: Volume No. 29(6) Page 1898