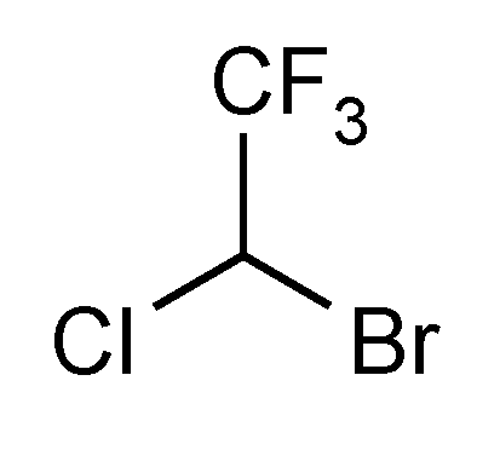
Halothane
Ethane, 2-bromo-2-chloro-1,1,1-trifluoro-, (±)-.
(±)-2-Bromo-2-chloro-1,1,1-trifluoroethane
» Halothane contains not less than 0.008 percent and not more than 0.012 percent of thymol, by weight, as a stabilizer.
Packaging and storage—
Preserve in tight, light-resistant containers, preferably of Type NP glass, and avoid exposure to excessive heat. Dispense it only in the original container.
Specific gravity  841
841 :
between 1.872 and 1.877 at 20
:
between 1.872 and 1.877 at 20 .
.
Distilling range, Method II  721
721 —
Not less than 95% distills within a 1
—
Not less than 95% distills within a 1 range between 49
range between 49 and 51
and 51 , and not less than 100% distills between 49
, and not less than 100% distills between 49 and 51
and 51 , a correction factor of 0.040
, a correction factor of 0.040 per mm being applied as necessary.
per mm being applied as necessary.
Refractive index  831
831 :
between 1.369 and 1.371 at 20
:
between 1.369 and 1.371 at 20 .
.
Acidity or alkalinity—
Shake 20 mL with 20 mL of carbon dioxide-free water for 3 minutes, and allow the layers to separate: the aqueous layer requires not more than 0.1 mL of 0.010 N sodium hydroxide or not more than 0.6 mL of 0.010 N hydrochloric acid for neutralization, bromocresol purple TS being used as the indicator.
Water, Method I  921
921 :
not more than 0.03%.
:
not more than 0.03%.
Limit of nonvolatile residue—
Evaporate 50 mL in a tared dish on a steam bath to dryness, and dry the residue at 105 for 2 hours: the weight of the residue does not exceed 1 mg.
for 2 hours: the weight of the residue does not exceed 1 mg.
Chloride and bromide—
Shake 25 mL with 25 mL of water for 5 minutes, and allow the liquids to separate completely. Draw off the water layer, and to 10 mL add 1 drop of nitric acid and 5 drops of silver nitrate TS: no opalescence is produced.
Thymol content—
Standard thymol solution—
Prepare a standard solution of thymol in 0.25 N sodium hydroxide containing 0.1 mg of thymol per mL.
Buffer solution—
Use pH 8.0 alkaline borate buffer (see under Solutions in the section Reagents, Indicators, and Solutions).
Chlorimide solution—
Dissolve 100 mg of 2,6-dibromoquinonechlorimide in 25 mL of dehydrated alcohol. Prepare a fresh solution for each assay.
Standard thymol curve—
Pipet into three 100-mL volumetric flasks 1 mL, 3 mL, and 5 mL, respectively, of Standard thymol solution, and add 0.25 N sodium hydroxide to make the final volume 5.0 mL. Add 5.0 mL of 0.25 N sodium hydroxide to a fourth flask in which the blank is to be prepared. To each flask add 10 mL of Buffer solution, mix by gentle swirling, and add 1 mL of Chlorimide solution. Allow to stand for 15 minutes, accurately timed, add 3 mL of 0.25 N sodium hydroxide to each flask, and add water to volume. With a suitable spectrophotometer, measure the absorbances of the thymol-containing solutions relative to the blank at 590 nm. Plot the readings and draw the curve of best fit.
Procedure—
Place about 2 mL of Halothane, accurately weighed, in a 100-mL volumetric flask containing 5 mL of 0.25 N sodium hydroxide, and mix by gentle swirling. Evaporate the halothane under a stream of nitrogen, and add 10 mL of Buffer solution and 1 mL of Chlorimide solution. Swirl gently, allow to stand for 15 minutes, accurately timed, add 3 mL of 0.25 N sodium hydroxide, and add water to volume. Read the absorbance of the resulting solution, and by reference to the Standard thymol curve, calculate the percentage of thymol in the weight of Halothane taken.
Chromatographic purity—
Standard preparation—
Add 1.0 µL of 1,1,2-trichloro-1,2,2-trifluoroethane to 20.0 mL of the test specimen.
Chromatographic system—
Under typical conditions, the gas chromatograph is equipped with a flame-ionization detector, and contains a 3-m × 2-mm stainless steel column packed with 20% G24 on support S1AB. The column is maintained at 60 , the injection port and detector block are maintained at 200
, the injection port and detector block are maintained at 200 , and nitrogen is used as the carrier gas at a flow rate of about 15 mL per minute. Typical retention times are about 5 minutes for 1,1,2-trichloro-1,2,2-trifluoroethane and about 13 minutes for halothane.
, and nitrogen is used as the carrier gas at a flow rate of about 15 mL per minute. Typical retention times are about 5 minutes for 1,1,2-trichloro-1,2,2-trifluoroethane and about 13 minutes for halothane.
Procedure—
Inject separately equal volumes (about 2 µL) of the Standard preparation and Halothane into a suitable gas chromatograph, and record the chromatograms. The total area of all peaks (except that of halothane) recorded for the test specimen does not exceed that due to the added 1,1,2-trichloro-1,2,2-trifluoroethane in the Standard preparation (0.005%).
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Daniel K. Bempong, Ph.D.
Senior Scientist 1-301-816-8143 |
(MDPS05) Monograph Development-Pulmonary and Steroids |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 2549
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.