Ergoloid Mesylates
C35H41N5O5·CH4O3S (dihydroergocristine mesylate)




 707.84
707.84
C32H43N5O5·CH4O3S (dihydro-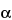 -ergocryptine mesylate)
-ergocryptine mesylate)




 673.82
673.82
C32H43N5O5·CH4O3S (dihydro-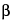 -ergocryptine mesylate)
-ergocryptine mesylate)




 673.82
673.82
Ergotaman-3¢,6¢,18-trione, 9,10-dihydro-12¢-hydroxy-2¢,5¢-bis(1-methylethyl)-, (5¢
Dihydroergotoxine monomethanesulfonate (salt).
An equiproportional mixture of dihydroergocornine mesylate, dihydroergocristine mesylate, and ratio of dihydro-
» Ergoloid Mesylates is a mixture of the methanesulfonate salts of the three hydrogenated alkaloids, dihydroergocristine (C35H41N5O5·CH4O3S), dihydroergocornine (C31H41N5O5·CH4O3S), and dihydroergocryptine (C32H43N5O5·CH4O3S), in an approximate weight ratio of 1:1:1. Ergoloid Mesylates contains not less than 97.0 percent and not more than 103.0 percent of the alkaloid methanesulfonate mixture, calculated on the anhydrous basis, and not less than 30.3 percent and not more than 36.3 percent of the methanesulfonate salt of each of the individual alkaloids. Dihydroergocryptine mesylate exists as a mixture of alpha- and beta- isomers. The ratio of alpha- to beta- isomers is not less than 1.5:1.0 and not more than 2.5:1.0.
Packaging and storage—
Preserve in tight, light-resistant containers.
Identification—
A:
The IR absorption spectrum of a potassium bromide dispersion of it exhibits maxima only at the same wavelengths as that of a similar, undried preparation of USP Ergoloid Mesylates RS.
B:
In a suitable chromatographic chamber, arranged for thin-layer chromatography, place a volume of a solvent system consisting of a mixture of acetone, n-butyl alcohol, ammonium hydroxide, and water (65:20:10:5) sufficient to develop the chromatogram. Prepare a test solution of Ergoloid Mesylates in a mixture of chloroform and methanol (9:1) containing 40 mg per mL. Apply 10 µL of this solution and 10 µL of a reference solution of methanesulfonic acid containing 0.4 mL in 100 mL of a mixture of chloroform and methanol (9:1) to a suitable thin-layer chromatographic plate (see Chromatography  621
621 ) coated with a 0.25-mm layer of chromatographic silica gel. Develop the chromatogram until the solvent front has moved 10 cm. Remove the plate from the developing chamber, mark the solvent front, and dry in a current of cold air. Spray the plate with a 1 in 1000 solution of bromocresol purple in alcohol that previously has been adjusted to the purple color with 6 N ammonium hydroxide, then place in a stream of warm air until the spots appear: the RF value of the methanesulfonic acid spot obtained from the test solution corresponds to that obtained from the reference solution.
) coated with a 0.25-mm layer of chromatographic silica gel. Develop the chromatogram until the solvent front has moved 10 cm. Remove the plate from the developing chamber, mark the solvent front, and dry in a current of cold air. Spray the plate with a 1 in 1000 solution of bromocresol purple in alcohol that previously has been adjusted to the purple color with 6 N ammonium hydroxide, then place in a stream of warm air until the spots appear: the RF value of the methanesulfonic acid spot obtained from the test solution corresponds to that obtained from the reference solution.
Specific rotation  781S
781S :
between +11.0
:
between +11.0 and +15.0
and +15.0 .
.
Test solution:
10 mg per mL, in dilute alcohol (1 in 2).
pH  791
791 :
between 4.2 and 5.2 in a solution (1 in 200).
:
between 4.2 and 5.2 in a solution (1 in 200).
Water, Method I  921
921 :
not more than 5.0%.
:
not more than 5.0%.
Limit of ergotamine—
Prepare three solutions in a mixture of chloroform and methanol (9:1) containing 5 mg of Ergoloid Mesylates per mL, 5 mg of USP Ergoloid Mesylates RS per mL, and 5 mg of Ergotamine Tartrate per mL. Apply 5-µL volumes of the solutions at points about 2 cm from the bottom edge of a thin-layer chromatographic plate (see Chromatography  621
621 ) coated with a 0.25-mm layer of chromatographic silica gel mixture, and allow the spots to dry. Add the solvent system consisting of a mixture of chloroform and methanol (9:1) and a small beaker of ammonium hydroxide to a suitable chamber, seal, and allow to equilibrate for 30 minutes. Develop the chromatogram in the equilibrated chamber until the solvent front has moved about 15 cm from the points of application. Remove the plate, air-dry, and locate the spots, first by viewing under long-wavelength UV light, and then by spraying with a reagent prepared by dissolving 800 mg of p-(dimethylamino)benzaldehyde in a mixture of 80 mL of alcohol and 11 mL of sulfuric acid: the chromatogram from Ergoloid Mesylates shows primary spots that correspond in size and color to the spots obtained from the USP Ergoloid Mesylates RS solution, and shows no spot corresponding to the principal spot in the chromatogram of Ergotamine Tartrate.
) coated with a 0.25-mm layer of chromatographic silica gel mixture, and allow the spots to dry. Add the solvent system consisting of a mixture of chloroform and methanol (9:1) and a small beaker of ammonium hydroxide to a suitable chamber, seal, and allow to equilibrate for 30 minutes. Develop the chromatogram in the equilibrated chamber until the solvent front has moved about 15 cm from the points of application. Remove the plate, air-dry, and locate the spots, first by viewing under long-wavelength UV light, and then by spraying with a reagent prepared by dissolving 800 mg of p-(dimethylamino)benzaldehyde in a mixture of 80 mL of alcohol and 11 mL of sulfuric acid: the chromatogram from Ergoloid Mesylates shows primary spots that correspond in size and color to the spots obtained from the USP Ergoloid Mesylates RS solution, and shows no spot corresponding to the principal spot in the chromatogram of Ergotamine Tartrate.
Limit of nonhydrogenated alkaloids—
Prepare a solution in alcohol containing 0.4 mg of Ergoloid Mesylates per mL, and prepare a 1 in 10 dilution of the first solution. Determine the absorbances in 1-cm cells of the first solution at 317.5 nm and the dilution at 280 nm, using alcohol as the blank: the absorbance of the first solution is not more than 0.15 times that of the dilution.
Assay—
Mobile phase—
Prepare a degassed solution containing a mixture of water, acetonitrile, and triethylamine (80:20:2.5). Adjust the ratio as necessary.
Standard preparation—
Transfer about 10 mg of USP Ergoloid Mesylates RS, accurately weighed, to a 10-mL volumetric flask. Dissolve in a mixture of acetonitrile and water (1:1), dilute with the same solvent to volume, and mix. Prepare this solution fresh.
Assay preparation—
Using about 10 mg of Ergoloid Mesylates, accurately weighed, proceed as directed for Standard preparation.
Chromatographic system
(see Chromatography  621
621 )—The liquid chromatograph is equipped with a 280-nm detector and a 4-mm × 30-cm column that contains packing L1. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the resolution, R, between dihydro-
)—The liquid chromatograph is equipped with a 280-nm detector and a 4-mm × 30-cm column that contains packing L1. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the resolution, R, between dihydro-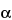 -ergocryptine mesylate and dihydroergocristine mesylate is not less than 1.35, the resolution, R, between dihydroergocristine and dihydro-
-ergocryptine mesylate and dihydroergocristine mesylate is not less than 1.35, the resolution, R, between dihydroergocristine and dihydro-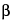 -ergocryptine is not less than 1.0; the column efficiency determined for the dihydro-
-ergocryptine is not less than 1.0; the column efficiency determined for the dihydro-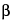 -ergocryptine mesylate peak is not less than 950 theoretical plates; the tailing factor for dihydro-
-ergocryptine mesylate peak is not less than 950 theoretical plates; the tailing factor for dihydro-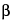 -ergocryptine mesylate is not more than 2.5; and the relative standard deviation of the sum of the four peaks for replicate injections is not more than 1.5%.
-ergocryptine mesylate is not more than 2.5; and the relative standard deviation of the sum of the four peaks for replicate injections is not more than 1.5%.
Procedure—
Separately inject equal volumes (about 20 µL) of the Standard preparation and the Assay preparation into the chromatograph by means of a suitable microsyringe or sampling valve, record the chromatograms, and measure the responses for the major peaks. The order of elution is dihydroergocornine, dihydro-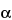 -ergocryptine, dihydroergocristine, and dihydro-
-ergocryptine, dihydroergocristine, and dihydro-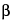 -ergocryptine. Calculate the total quantity, in mg, of these alkaloids in the portion of Ergoloid Mesylates taken by the formula:
-ergocryptine. Calculate the total quantity, in mg, of these alkaloids in the portion of Ergoloid Mesylates taken by the formula:
10C(rU / rS)
in which C is the concentration, in mg per mL, of USP Ergoloid Mesylates RS in the Standard preparation; and rU and rS are the sums of the responses of the four major peaks obtained from the Assay preparation and the Standard preparation, respectively.
Calculate the percentage of each alkaloid taken by the formula:
100ri(MW)i / S[ri(MW)i]
in which ri is the peak response of an individual alkaloid; (MW)i is the molecular weight of that alkaloid; and S[ri(MW)i] is the summation of the products of peak responses and molecular weights calculated for the four alkaloids.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Ravi Ravichandran, Ph.D.
Senior Scientist 1-301-816-8330 |
(MDPP05) Monograph Development-Psychiatrics and Psychoactives |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 2271
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.
