Echinacea purpurea Root
» Echinacea purpurea Root consists of the dried rhizome and roots of Echinacea purpurea (L.) Moench (Fam. Asteraceae). It is harvested in the fall after 3 or more years of growth. It contains not less than 0.5 percent of total phenols, calculated on the dried basis as the sum of caftaric acid (C13H12O9), chicoric acid (C22H18O12), chlorogenic acid (C16H18O9), and echinacoside (C35H46O20). It contains not less than 0.025 percent of alkamides calculated as dodecatetraenoic acid isobutylamides (C16H25NO).
Packaging and storage—
Store in well-closed, light-resistant containers.
Labeling—
The label states the Latin binomial and, following the official name, the parts of the plant contained in the article.
USP Reference standards  11
11 —
—
USP Chlorogenic Acid RS.
USP Powdered Echinacea purpurea Extract RS. USP 2E,4E-Hexadienoic Acid Isobutylamide RS.
USP Chlorogenic Acid RS.
USP Powdered Echinacea purpurea Extract RS. USP 2E,4E-Hexadienoic Acid Isobutylamide RS.
Botanic characteristics—
Macroscopic—
The roots are cylindrical and irregularly branched. The outer surface is dark brown and longitudinally striated; fractures are short and tough. Transverse sections show a thin periderm and yellowish xylem with distinct rays. In older roots, the pith is spongy, with a brownish center surrounded by yellow.
Microscopic—
Rhizomes and roots in transverse section show a thin outer bark separated from a wide xylem by a brown vascular cambium. The cork is composed of several rows of thin-walled cells containing brown pigment. Schizogenous resin canals are present in the cortex. The rhizome contains bast fibers and stone cells. The xylem, with distinct rays, contains tracheary elements composed of reticulated vessels and tracheids (about 80 × 30 µm) with bordered pits and slanted end walls. Vessels and tracheids are surrounded by thick-walled parenchyma and fibers; fibers are elongated with narrow lumens and funnel-shaped ends (20 to 40 µm wide). Polygonal sclereids (about 50 µm in diameter) are also present. Xylem fibers have minimal or no phytomelanin deposits (unlike Echinacea angustifolia and Echinacea pallida). A melanogenic layer is present between adjacent xylem parenchyma cell walls. The rhizome, with pith, is composed of pitted parenchyma cells containing inulin crystals. Starch is minimal to absent, and calcium oxalate crystals are absent.
Identification—
A:
Thin-Layer Chromatographic Identification Test  201
201 —
—
presence of chicoric acid and absence of echinacoside—
Test solution—
Prepare as directed for Identification test A under Echinacea angustifolia, except to use Echinacea purpurea Root instead of Echinacea angustifolia.
Standard solution 1—
Proceed as directed for Identification test A under Echinacea angustifolia, except to use USP Powdered Echinacea purpurea Extract RS instead of USP Powdered Echinacea angustifolia Extract RS.
Standard solution 2 , Developing solvent system, Spray reagent 1, and Spray reagent 2—
Prepare as directed for Identification test A under Echinacea angustifolia.
Procedure—
Proceed as directed in the chapter. Develop the chromatograms in Developing solvent system until the solvent front has moved not less than 18 cm, and dry the plate in a current of air. Spray the plate with Spray reagent 1 followed by Spray reagent 2, and examine the plate under UV light at 365 nm: the chromatogram obtained from the Test solution shows a yellowish-green zone at an RF value of 0.75 due to chicoric acid and another yellowish-green zone at an RF value of 0.45 due to caftaric acid, both zones corresponding in color and RF value to zones in the chromatogram obtained from Standard solution 1. The chromatogram obtained from the Test solution does not show or shows only traces of a zone at an RF value of 0.1 due to echinacoside (present in Echinacea angustifolia and in Echinacea pallida) that corresponds to a yellowish spot in the chromatogram obtained from Standard solution 1, and does not show a zone that corresponds in color and RF value to the spot for 1,3-dicaffeoylquinic acid (cynarin) (present in Echinacea angustifolia) in the chromatogram obtained from Standard solution 2. Other colored zones of varying intensities may be observed in the chromatogram obtained from the Test solution.
B: Thin-Layer Chromatographic Identification Test  201
201 —
—
presence of isobutylalkenylamides—
Test solution—
Proceed as directed for Identification test B under Echinacea angustifolia, except to use the chloroform extract retained from Identification test A under Echinacea purpurea Root instead of the chloroform extract retained from Identification test A under Echinacea angustifolia.
Standard solution 1—
Dissolve an accurately weighed quantity of USP Echinacea purpurea Extract RS in methanol to obtain a solution having a known concentration of about 100 mg per mL.
Standard solution 2, Developing solvent system, and Spray reagent—
Proceed as directed for Identification test B under Echinacea angustifolia.
Procedure—
Proceed as directed in the chapter. Develop the chromatograms in Developing solvent system until the solvent front has moved not less than 12 cm, and dry the plate in a current of air. Examine the plate under UV light at 254 nm: the chromatogram obtained from the Test solution shows one main zone corresponding in RF value to the zone due to dodeca-2E,4E,8Z,10E-tetraenoic acid isobutylamide and dodeca-2E,4E,8Z,10Z-tetraenoic acid isobutylamide in the chromatogram of Standard solution 1 and below this zone there are several other zones due to  ,
, ,
,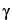 ,
,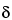 -unsaturated isobutylamides. Spray the plate with Spray reagent, and then heat the plate at 100
-unsaturated isobutylamides. Spray the plate with Spray reagent, and then heat the plate at 100 for 5 minutes. Examine the plate under long-wavelength UV light: the zone due to dodeca-2E,4E,8Z,10E-tetraenoic acid isobutylamide and dodeca-2E,4E,8Z,10Z-tetraenoic acid isobutylamide turns blue-black, and below this zone there are several other zones due to
for 5 minutes. Examine the plate under long-wavelength UV light: the zone due to dodeca-2E,4E,8Z,10E-tetraenoic acid isobutylamide and dodeca-2E,4E,8Z,10Z-tetraenoic acid isobutylamide turns blue-black, and below this zone there are several other zones due to  ,
, ,
,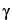 ,
,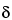 -unsaturated isobutylamides (not detectable in Echinacea pallida) that turn violet (unlike the corresponding zones in the chromatogram of Echinacea angustifolia that are mostly yellowish due to
-unsaturated isobutylamides (not detectable in Echinacea pallida) that turn violet (unlike the corresponding zones in the chromatogram of Echinacea angustifolia that are mostly yellowish due to  ,
, -unsaturated isobutylamides). A zone due to
-unsaturated isobutylamides). A zone due to  -sitosterol that corresponds in RF value to the principal spot in the chromatogram of Standard solution 2 is also observed.
-sitosterol that corresponds in RF value to the principal spot in the chromatogram of Standard solution 2 is also observed.
C:
The retention times for the relevant peaks in the chromatogram of the Test solution, mainly due to caftaric acid and chicoric acid, correspond to those in the chromatogram of Standard solution 1, as obtained in the test for Content of total phenols. An echinacoside peak is not detectable or is very weak.
Content of total phenols—
Solvent, Solution A, Solution B, Mobile phase, and Standard solution 2—
Proceed as directed in the test for Content of total phenols under Echinacea angustifolia.
Standard solution 1—
Dissolve an accurately weighed quantity of USP Powdered Echinacea purpurea Extract RS in Solvent, shaking for 1 minute, and dilute with Solvent to obtain a solution having a known concentration of about 5 mg per mL. Pass through a membrane filter having a 0.45-µm or finer porosity.
Test solution—
Proceed as directed for Content of phenols under Echinacea angustifolia, except to use finely powdered Echinacea purpurea Root instead of Echinacea angustifolia.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 330-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L1. The column temperature is maintained at 35
)—
The liquid chromatograph is equipped with a 330-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L1. The column temperature is maintained at 35 . The flow rate is about 1.5 mL per minute. The chromatograph is programmed as follows:
. The flow rate is about 1.5 mL per minute. The chromatograph is programmed as follows:
Chromatograph Standard solution 1, and record the peak responses as directed for Procedure: the chromatogram obtained is similar to the Reference Chromatogram for total phenols provided with USP Powdered Echinacea purpurea Extract RS. Chromatograph Standard solution 2, and record the responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 2%.
| Time (minutes) |
Solution A
(%) |
Solution B
(%) |
Elution |
| 0–13 | 90®78 | 10®22 | linear gradient |
| 13–14 | 78®60 | 22®40 | linear gradient |
| 14–17.5 | 60 | 40 | isocratic |
| 17.5–18 | 60®90 | 40®10 | linear gradient |
| 18–30 | 90 | 10 | equilibration |
Procedure—
Proceed as directed in the test for Content of total phenols under Echinacea angustifolia. Separately calculate the percentage of caftaric acid (C13H12O9), chicoric acid (C22H18O12), chlorogenic acid (C16H18O9), and echinacoside (C35H46O20) in the portion of Echinacea purpurea Root taken by the formula:
2500F(C/W)(ri / rS)
in which F is the response factor and is equal to 0.695 for chicoric acid, 0.881 for caftaric acid, 1.000 for chlorogenic acid, and 2.220 for echinacoside; C is the concentration, in mg per mL, of USP Chlorogenic Acid RS in Standard solution 2; W is the weight, in mg, of Echinacea purpurea Root taken; and ri and rS are the peak responses for the relevant analyte obtained from the Test solution and Standard solution 2, respectively. Calculate the percentage of total phenols in the portion of Echinacea purpurea Root taken by adding the individual percentages calculated.
Content of alkamides—
Mobile phase and Standard solution 2—
Proceed as directed in the test for Content of dodecatetraenoic acid isobutylamides under Echinacea angustifolia.
Standard solution 1—
Proceed as directed for Content of dodecatetraenoic acid isobutylamides under Echinacea angustifolia, except to use USP Powdered Echinacea purpurea Extract RS instead of USP Powdered Echinacea angustifolia Extract RS.
Test solution—
Proceed as directed for Content of dodecatetraenoic acid isobutylamides under Echinacea angustifolia, except to use Echinacea purpurea Root instead of Echinacea angustifolia.
Chromatographic system (see Chromatography  621
621 )—
Proceed as directed for Content of dodecatetraenoic acid isobutylamides under Echinacea angustifolia, except to use the Reference Chromatogram for alkamides provided with USP Powdered Echinacea purpurea Extract RS instead of the Reference Chromatogram provided with USP Powdered Echinacea angustifolia Extract RS.
)—
Proceed as directed for Content of dodecatetraenoic acid isobutylamides under Echinacea angustifolia, except to use the Reference Chromatogram for alkamides provided with USP Powdered Echinacea purpurea Extract RS instead of the Reference Chromatogram provided with USP Powdered Echinacea angustifolia Extract RS.
Procedure—
Proceed as directed in the test for Content of dodecatetraenoic acid isobutylamides under Echinacea angustifolia. Identify the peaks of the ten major alkamides in the chromatogram obtained from the Test solution by comparison with the chromatogram obtained from Standard solution 1. Calculate the percentage of alkamides in the portion of Echinacea purpurea Root taken by the formula:
10(1.353)(C/W)(ri / rS)
in which 1.353 is the response factor for 2E,4E-hexadienoic acid isobutylamide; C is the concentration, in mg per mL, of USP 2E,4E-Hexadienoic Acid Isobutylamide RS in Standard solution 2; W is the weight, in g, of Echinacea purpurea Root taken; ri is the sum of the peak responses of the relevant analytes obtained from the Test solution; and rS is the peak response obtained from Standard solution 2.
Other requirements—
It meets the requirements of the tests for Loss on drying, Foreign organic matter, Total ash, Acid-insoluble ash, Pesticide residues, and Heavy metals under Echinacea angustifolia.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Maged H. Sharaf, Ph.D.
Senior Scientist 1-301-816-8318 |
(DSB05) Dietary Supplements - Botanicals |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 999
Pharmacopeial Forum: Volume No. 30(2) Page 561
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.