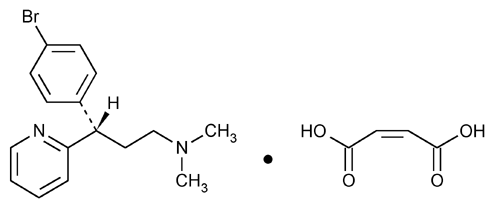
Dexbrompheniramine Maleate
2-Pyridinepropanamine,
(+)-2-[p-Bromo-
» Dexbrompheniramine Maleate contains not less than 98.0 percent and not more than 100.5 percent of C16H19BrN2·C4H4O4, calculated on the dried basis.
Packaging and storage—
Preserve in tight, light-resistant containers.
Identification—
B:
Ultraviolet Absorption  197U
197U —
—
Solution:
35 µg per mL.
Medium:
methanol.
Absorptivities at 261 nm, calculated on the dried basis, do not differ by more than 3.0%.
Loss on drying  731
731 —
Dry it at 65
—
Dry it at 65 for 4 hours: it loses not more than 0.5% of its weight.
for 4 hours: it loses not more than 0.5% of its weight.
Residue on ignition  281
281 :
not more than 0.2%.
:
not more than 0.2%.
Related compounds—
Test solution—
Dissolve about 200 mg of Dexbrompheniramine Maleate in 5 mL of methylene chloride, and mix.
Chromatographic system
(see Chromatography  621
621 )—The gas chromatograph is equipped with a flame-ionization detector and a 4-mm × 1.2-m glass column containing 3% phase G3 on support S1AB. The column temperature is maintained at about 190
)—The gas chromatograph is equipped with a flame-ionization detector and a 4-mm × 1.2-m glass column containing 3% phase G3 on support S1AB. The column temperature is maintained at about 190 , and the injection port and detector temperatures are both maintained at about 250
, and the injection port and detector temperatures are both maintained at about 250 . The carrier gas is dry helium, flowing at a rate adjusted to obtain a retention time of 6 to 7 minutes for the main peak. Chromatograph the Test solution, record the chromatogram, and determine the peak area as directed under Procedure: the tailing factor for the dexbrompheniramine maleate peak is not more than 1.8.
. The carrier gas is dry helium, flowing at a rate adjusted to obtain a retention time of 6 to 7 minutes for the main peak. Chromatograph the Test solution, record the chromatogram, and determine the peak area as directed under Procedure: the tailing factor for the dexbrompheniramine maleate peak is not more than 1.8.
Procedure—
Inject a volume (about 1 µL) of the Test solution into the chromatograph. Record the chromatogram for a total time of not less than twice the retention time of the dexbrompheniramine peak, and measure the areas of the peaks. The total relative area of all extraneous peaks (except that of the solvent peak and maleic acid, if observed) does not exceed 2.0%.
Assay—
Dissolve about 400 mg of Dexbrompheniramine Maleate, accurately weighed, in 50 mL of glacial acetic acid, add 1 drop of crystal violet TS, and titrate with 0.1 N perchloric acid VS to a green endpoint. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 21.77 mg of C16H19BrN2·C4H4O4.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Daniel K. Bempong, Ph.D.
Senior Scientist 1-301-816-8143 |
(MDPS05) Monograph Development-Pulmonary and Steroids |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 2093
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.