Cyclomethicone
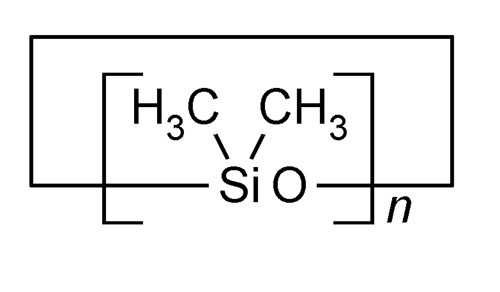
» Cyclomethicone is a fully methylated cyclic siloxane containing repeating units of the formula:
[–(CH3)2SiO–]n
in which n is 4, 5, or 6, or a mixture of them. It contains not less than 98.0 percent of (C2H6OSi)n, calculated as the sum of cyclomethicone 4, cyclomethicone 5, and cyclomethicone 6, and not less than 95.0 percent and not more than 105.0 percent of the labeled amount of any one or more of the individual cyclomethicone components.
Packaging and storage—
Preserve in tight containers.
Labeling—
Label it to state, as part of the official title, the n-value of the Cyclomethicone. Where it is a mixture of 2 or 3 such cyclic siloxanes, the label states the n-value and percentage of each in the mixture.
USP Reference standards  11
11 —
—
USP Cyclomethicone 4 RS.
USP Cyclomethicone 5 RS.
USP Cyclomethicone 6 RS.
USP Cyclomethicone 4 RS.
USP Cyclomethicone 5 RS.
USP Cyclomethicone 6 RS.
Identification—
Proceed as directed under  197S
197S , except to use neat liquids. The IR absorption spectrum, determined in a 0.1-mm cell, exhibits maxima only at the same wavelengths as that of a similar preparation of USP Cyclomethicone 4 RS, USP Cyclomethicone 5 RS, or USP Cyclomethicone 6 RS.
, except to use neat liquids. The IR absorption spectrum, determined in a 0.1-mm cell, exhibits maxima only at the same wavelengths as that of a similar preparation of USP Cyclomethicone 4 RS, USP Cyclomethicone 5 RS, or USP Cyclomethicone 6 RS.
Limit of nonvolatile residue—
Evaporate 2.0 g in an open, tared aluminum dish in a circulating air oven at 150 for 2 hours, allow to cool in a desiccator, and weigh: the weight of the residue so obtained does not exceed 3.0 mg, corresponding to not more than 0.15% (w/w).
for 2 hours, allow to cool in a desiccator, and weigh: the weight of the residue so obtained does not exceed 3.0 mg, corresponding to not more than 0.15% (w/w).
Assay—
The gas chromatograph is equipped with a thermal conductivity detector and a suitable recorder, and contains a 3.66-m × 3-mm column packed with 20% liquid phase G1 on 60- to 80-mesh packing S1A (see Gas Chromatography under Chromatography  621
621 ). The column is temperature-programmed at a rate of about 8
). The column is temperature-programmed at a rate of about 8 per minute from 125
per minute from 125 to 320
to 320 , the injection port is maintained at a temperature of about 300
, the injection port is maintained at a temperature of about 300 , and the detector block is maintained at a temperature of about 350
, and the detector block is maintained at a temperature of about 350 . Helium is used as the carrier gas, flowing at a rate of about 20 mL per minute. Separately inject about 1 µL of USP Cyclomethicone 4 RS, USP Cyclomethicone 5 RS, and USP Cyclomethicone 6 RS into the gas chromatograph, record the chromatograms, and note the retention times of the peaks. Similarly inject about 1 µL of Cyclomethicone, record the chromatogram, and measure the responses of the major peaks. Calculate the percentage of cyclomethicone 4, cyclomethicone 5, and cyclomethicone 6 by dividing 100 times the response of each peak at the retention time of the corresponding reference standard by the sum of all of the responses in the chromatogram. The percentages obtained from duplicate injections agree to within 1.0%. Calculate the percentage purity by adding the percentages of cyclomethicone 4, cyclomethicone 5, and cyclomethicone 6.
. Helium is used as the carrier gas, flowing at a rate of about 20 mL per minute. Separately inject about 1 µL of USP Cyclomethicone 4 RS, USP Cyclomethicone 5 RS, and USP Cyclomethicone 6 RS into the gas chromatograph, record the chromatograms, and note the retention times of the peaks. Similarly inject about 1 µL of Cyclomethicone, record the chromatogram, and measure the responses of the major peaks. Calculate the percentage of cyclomethicone 4, cyclomethicone 5, and cyclomethicone 6 by dividing 100 times the response of each peak at the retention time of the corresponding reference standard by the sum of all of the responses in the chromatogram. The percentages obtained from duplicate injections agree to within 1.0%. Calculate the percentage purity by adding the percentages of cyclomethicone 4, cyclomethicone 5, and cyclomethicone 6.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Elena Gonikberg, Ph.D.
Senior Scientist 1-301-816-8251 |
(MDGRE05) Monograph Development-Gastrointestinal Renal and Endocrine |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 1221
Pharmacopeial Forum: Volume No. 31(4) Page 1140
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.