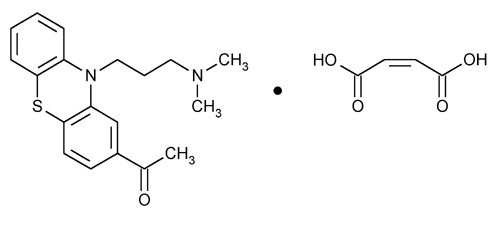
Acepromazine Maleate
Ethanone, 1-[10-[3-(dimethylamino)propyl]-10H-phenothiazin-2-yl]-, (Z)-2-butenedioate (1:1).
10-[3-(Dimethylamino)propyl]phenothiazin-2-yl methyl ketone maleate (1:1)
» Acepromazine Maleate contains not less than 98.0 percent and not more than 101.0 percent of C19H22N2OS·C4H4O4, calculated on the anhydrous basis.
Packaging and storage—
Preserve in well-closed containers, protected from light. Store at room temperature.
Labeling—
Label it to indicate that it is for veterinary use only.
USP Reference standards  11
11 —
—
USP Acepromazine Maleate RS.
USP Acepromazine Maleate RS.
note—Throughout the following procedures, protect test or assay specimens, the USP Reference Standard, and solutions containing them, by conducting the procedures without delay, under subdued light, or using low-actinic glassware.
Identification—
B:
The retention time of the major peak for acepromazine in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
pH  791
791 :
between 4.0 and 5.5, in a solution (1 in 100).
:
between 4.0 and 5.5, in a solution (1 in 100).
Water, Method I  921
921 :
not more than 1.0%.
:
not more than 1.0%.
Residue on ignition  281
281 :
not more than 0.2%.
:
not more than 0.2%.
Related compounds—
[note—Conduct this test without exposure to daylight, and with the minimum necessary exposure to artificial light.] Prepare a test solution of it in a mixture of methanol and diethylamine (19:1) containing 20.0 mg per mL. Prepare a Standard solution containing 0.1 mg per mL by quantitatively diluting 1 volume of the test solution with 200 volumes of the same solvent mixture. Apply separately 10-µL portions of the test solution and the Standard solution to the starting line of a suitable thin-layer chromatographic plate (see Chromatography  621
621 ) coated with a 0.25-mm layer of chromatographic silica gel mixture. Develop the chromatogram in a chromatographic chamber with a solvent system consisting of a mixture of n-heptane, isobutyl alcohol, and diethylamine (75:17:8) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the chamber, and allow it to air-dry. Examine the plate under short-wavelength UV light: no spot, other than the principal acepromazine spot and any at the origin, observed in the chromatogram obtained from the test solution is more intense than the principal spot observed in the chromatogram obtained from the Standard solution (0.5%).
) coated with a 0.25-mm layer of chromatographic silica gel mixture. Develop the chromatogram in a chromatographic chamber with a solvent system consisting of a mixture of n-heptane, isobutyl alcohol, and diethylamine (75:17:8) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the chamber, and allow it to air-dry. Examine the plate under short-wavelength UV light: no spot, other than the principal acepromazine spot and any at the origin, observed in the chromatogram obtained from the test solution is more intense than the principal spot observed in the chromatogram obtained from the Standard solution (0.5%).
Assay—
Mobile phase—
Add 6 mL of triethylamine to 700 mL of water, and adjust with phosphoric acid to a pH of 2.5. Mix 700 mL of this solution and 300 mL of acetonitrile, and degas. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard preparation—
Transfer about 50 mg of USP Acepromazine Maleate RS, accurately weighed, to a 50-mL volumetric flask, dissolve in 0.05 N hydrochloric acid, dilute with 0.05 N hydrochloric acid to volume, and mix. Transfer 5.0 mL of this solution to a second 50-mL volumetric flask, dilute with water to volume, and mix. This solution contains about 0.1 mg of USP Acepromazine Maleate RS per mL.
Assay preparation—
Transfer about 50 mg of Acepromazine Maleate, accurately weighed, to a 50-mL volumetric flask; dissolve in 0.05 N hydrochloric acid; dilute with 0.05 N hydrochloric acid to volume; and mix. Transfer 5.0 mL of this solution to a second 50-mL volumetric flask, dilute with water to volume, and mix.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 280-nm detector and a 4-mm × 15-cm column that contains 5-µm packing L7. The flow rate is about 1 mL per minute. Chromatograph the Standard preparation, and record the responses as directed for Procedure: the column efficiency is not less than 1500 theoretical plates; the tailing factor is not more than 2.5; and the relative standard deviation for replicate injections is not more than 2.0%.
)—
The liquid chromatograph is equipped with a 280-nm detector and a 4-mm × 15-cm column that contains 5-µm packing L7. The flow rate is about 1 mL per minute. Chromatograph the Standard preparation, and record the responses as directed for Procedure: the column efficiency is not less than 1500 theoretical plates; the tailing factor is not more than 2.5; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the areas for the major peaks. Calculate the quantity, in mg, of acepromazine maleate (C19H22N2OS·C4H4O4) in the portion of Acepromazine Maleate taken by the formula:
500C(rU / rS)
in which C is the concentration, in mg per mL, of USP Acepromazine Maleate RS taken in the Standard preparation; and rU and rS are the acepromazine peak areas obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Ian DeVeau, Ph.D.
Director, Veterinary Drugs and Radiopharmaceuticals 1-301-816-8178 |
(VET05) Veterinary Drugs 05 |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 1386
Pharmacopeial Forum: Volume No. 29(6) Page 1832
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.