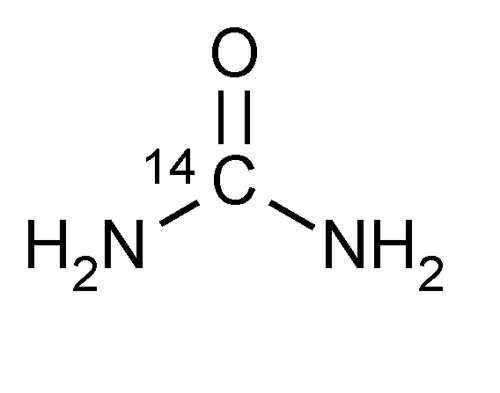
Urea C 14 Capsules
» Urea C 14 Capsules contain 14CH4N2O in which a portion of the molecules are labeled with radioactive 14C to provide 0.04 MBq (or 1 µCi) of radioactivity per capsule. It contains not less than 90.0 percent and not more than 110.0 percent of the labeled amount of 14C expressed as MBq (or µCi).
Packaging and storage—
Preserve in tight containers, and store at controlled room temperature.
Expiration date—
The expiration date is not later than two years from the date of manufacture.
Labeling—
Label it to include the following: the amount of 14C, expressed in MBq (or µCi) per Capsule at the time of calibration; the expiration date; the total radioactivity per container; and the statement, “Caution—Radioactive material.”
Radionuclide identification  821
821 —
A solution of 1 or more Capsules in 1 N hydrochloric acid when tested using a liquid scintillation counter produces beta emission having a 49 keV mean and a 156 keV max.
—
A solution of 1 or more Capsules in 1 N hydrochloric acid when tested using a liquid scintillation counter produces beta emission having a 49 keV mean and a 156 keV max.
Dissolution  711
711 —
—
Medium:
simulated gastric fluid TS; 500 mL.
Apparatus 1:
50 rpm.
Time:
10 minutes.
Procedure—
Determine the background levels of 14C with a 1-mL portion of the solution under test using a liquid scintillation counter.
Tolerances:
not less than 80% (Q) of the labeled amount of 14C is dissolved in 10 minutes.
Uniformity of dosage units  905
905 :
meet the requirements.
:
meet the requirements.
Radionuclidic purity  821
821 —
Determine the radionuclidic purity of a solution of 1 or more Capsules in water using a liquid scintillation counter: not less than 99.9% of the radioactivity is present as C 14.
—
Determine the radionuclidic purity of a solution of 1 or more Capsules in water using a liquid scintillation counter: not less than 99.9% of the radioactivity is present as C 14.
Radiochemical purity—
Adsorbent:
0.25-mm layer of chromatographic cellulose.
Test solution—
Open 2 Capsules and place them in a suitable container, add 8 mL of methanol, and mix.
Reference solution:
40 mg of urea per mL, in water.
Application volume:
20 µL of the Test solution and 4 µL of the Reference solution.
Developing solvent system:
n-butanol saturated with water.
Procedure—
Proceed as directed for Thin-Layer Chromatography under Chromatography  621
621 . Locate the spots on the plate by spraying with Ehrlich's reagent. Determine the radioactivity distribution with a suitable radiation detector (see Radioactivity
. Locate the spots on the plate by spraying with Ehrlich's reagent. Determine the radioactivity distribution with a suitable radiation detector (see Radioactivity  821
821 ), and obtain the RF value: the RF value of the principal spot from the Test solution corresponds to that obtained from the Reference solution, and the radioactivity of the 14C band is not less than 90% of the total radioactivity.
), and obtain the RF value: the RF value of the principal spot from the Test solution corresponds to that obtained from the Reference solution, and the radioactivity of the 14C band is not less than 90% of the total radioactivity.
Assay for radioactivity  821
821 —
Prepare a solution of 1 or more Capsules in 1 N hydrochloric acid. Using a liquid scintillation counter, determine the radioactivity, in MBq (or mCi) per mL by use of a calibrated system.
—
Prepare a solution of 1 or more Capsules in 1 N hydrochloric acid. Using a liquid scintillation counter, determine the radioactivity, in MBq (or mCi) per mL by use of a calibrated system.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Ian DeVeau, Ph.D.
Director, Veterinary Drugs and Radiopharmaceuticals 1-301-816-8178 |
(RMI05) Radiopharmaceuticals and Medical Imaging Agents 05 |
| Margareth R.C. Marques, Ph.D.
Senior Scientist 1-301-816-8106 |
(BPC05) Biopharmaceutics05 |
USP32–NF27 Page 1798
Pharmacopeial Forum: Volume No. 27(2) Page 2126