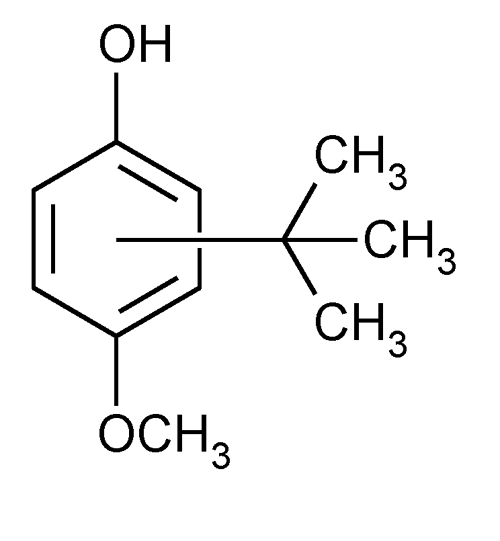
Butylated Hydroxyanisole
» Butylated Hydroxyanisole contains not less than 98.5 percent of C11H16O2.
Packaging and storage—
Preserve in well-closed containers.
USP Reference standards  11
11 —
—
USP 3-tert-Butyl-4-hydroxyanisole RS.
USP 2-tert-Butyl-4-hydroxyanisole RS.
USP 3-tert-Butyl-4-hydroxyanisole RS.
USP 2-tert-Butyl-4-hydroxyanisole RS.
Identification—
To 5 mL of a 1 in 10,000 solution in 72% alcohol add 2 mL of sodium borate solution (1 in 50) and 1 mL of a 1 in 10,000 solution of 2,6-dichloroquinone-chlorimide in dehydrated alcohol, and mix: a blue color is produced.
Residue on ignition  281
281 :
not more than 0.01%, determined on a 10-g specimen.
:
not more than 0.01%, determined on a 10-g specimen.
Heavy metals, Method II  231
231 :
0.001%.
:
0.001%.
Assay—
Internal standard solution—
Dissolve about 500 mg of 4-tert-butylphenol, accurately weighed, in acetone in a 100-mL volumetric flask, add acetone to volume, and mix.
Standard preparation—
Dissolve accurately weighed quantities of USP 3-tert-Butyl-4-hydroxyanisole RS and USP 2-tert-Butyl-4-hydroxyanisole RS in Internal standard solution to obtain a solution having known concentrations of about 9 mg per mL and about 1 mg per mL, respectively.
Assay preparation—
Dissolve about 100 mg of Butylated Hydroxyanisole, accurately weighed, in Internal standard solution in a 10-mL volumetric flask, dilute with Internal standard solution to volume, and mix.
Chromatographic system (see Chromatography  621
621 )—
The gas chromatograph is equipped with a flame-ionization detector, and contains a 2-mm × 1.8-m stainless-steel column packed with 10% liquid phase G26 on support S1A. The carrier gas is helium. The column is maintained isothermally at a temperature between 175
)—
The gas chromatograph is equipped with a flame-ionization detector, and contains a 2-mm × 1.8-m stainless-steel column packed with 10% liquid phase G26 on support S1A. The carrier gas is helium. The column is maintained isothermally at a temperature between 175 and 185
and 185 . The injection port temperature is maintained at 200
. The injection port temperature is maintained at 200 , and the detector temperature is maintained at about 250
, and the detector temperature is maintained at about 250 . Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the resolution, R, between the 3-tert-butyl-4-hydroxyanisole isomer and the 2-tert-butyl-4-hydroxyanisole isomer is not less than 1.3; the tailing factor is not more than 2.0; and the relative standard deviation is not more than 2.0% determined from the 3-tert-butyl-4-hydroxyanisole isomer and not more than 6.0% determined from the 2-tert-butyl-4-hydroxyanisole isomer.
. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the resolution, R, between the 3-tert-butyl-4-hydroxyanisole isomer and the 2-tert-butyl-4-hydroxyanisole isomer is not less than 1.3; the tailing factor is not more than 2.0; and the relative standard deviation is not more than 2.0% determined from the 3-tert-butyl-4-hydroxyanisole isomer and not more than 6.0% determined from the 2-tert-butyl-4-hydroxyanisole isomer.
Procedure—
Separately inject equal volumes (about 5 µL) of the Standard preparation and the Assay preparation into the gas chromatograph, record the chromatograms, and measure the peak areas for each isomer and the internal standard. Calculate the quantity, in mg, of each isomer in the portion of Butylated Hydroxyanisole taken by the formula:
10C(RU / RS)
in which C is the concentration, in mg per mL, of the appropriate USP Reference Standard; and RU and RS are the peak area ratios of the corresponding isomer to the internal standard obtained from the Assay preparation and the Standard preparation, respectively. Calculate the weight, in mg, of C11H16O2 in the portion of Butylated Hydroxyanisole taken by adding the quantities of the two isomers.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Robert H. Lafaver, B.A.
Scientist 1-301-816-8335 |
(EM105) Excipient Monographs 1 |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 1178
Pharmacopeial Forum: Volume No. 27(3) Page 2590
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.