This general chapter provides information about several aspects of botanical articles not covered in USP standards monographs. Although the standards in the monographs address the quality issues associated with botanical plant materials, extracts, and preparations of Pharmacopeial articles, there is a need to develop appropriate information to optimize the preharvesting conditions for appropriate growth and the postharvesting handling to achieve consistent quality with minimum variations in the composition of chemical constituents.
PROTOCOL CONTENTS
Black Cohosh (Actaea racemosa L.)
Ginger (Zingiber officinale Roscoe)
Valerian (Valeriana officinalis L.)
GENERAL GUIDANCES
It is recommened that, at a minimum, growers and others involved in the handling and distribution of botanical products should become familiar with and follow the WHO guidelines for good agricultural and collection practices for medicinal plants. Information about this document can be obtained at http://www.who.int/medicinedocs/collect/edmweb/pdf/s4928e/s4928e.pdf.
Commercial trade in natural products occurs in a global market. Material of domestic origin must be produced in compliance with all federal laws of the United States. Material of foreign origin, imported into the U.S., must be produced and transported in compliance with the laws of the U.S., the country of origin, and relevant international treaties. These include, but may not be limited to the following:
- The Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES) is an international agreement between governments. Its aim is to ensure that international trade in specimens of wild animals and plants does not threaten their survival. Information about CITES is available at http://www.cites.org.
- The Convention on Biological Diversity (CBD) establishes three main goals: the conservation of biological diversity, the sustainable use of its components, and the fair and equitable sharing of the benefits from the use of genetic resources. Each country that has ratified and is a party to the Convention is responsible for implementation by means of national enabling legislation that can differ from country to country.
- The Endangered Species Act (ESA) was originally adopted in 1973. The ESA is a law that aims to protect species of fish, wildlife, and plants believed to be threatened with extinction. The ESA is administered primarily by the U.S. Fish and Wildlife Service. Full text of the act is available at: http://endangered.fws.gov/esa.html.
Provided below is additional information not covered in the compendial specifications: compendial history; sources; collection and cultivation, including common adulterants; and drying, storing, and shipping. This information is provided to complement the standards for quality control in the monographs for botanical articles.
Compendial History—
The focus in this section is on historical compendial use that has strong validity, with only brief reference to anecdotal use. This is important information because traditional use is one of the elements taken into consideration to support the safety and the presumptions of benefits of botanical dietary supplements.
Sources—
Included here is the point of origin of the botanical and encompasses cultivation (defined as agricultural growing) and wildcrafting (defined as collected in the wild), along with a listing of the primary geographical (native) areas of production.
Collection and Cultivation—
This section discusses wildcrafting, the conservation of restricted and rare species, and the trend to cultivation as an ecological alternative; such optimal harvesting and collection practices serve to preserve the integrity of species and botanical products. It is subdivided into four subsections:
- Collection (conservation and ecology)
- Cultivation Practices
- Optimal Times for Harvest
- Optimal Handling and Processing Practices
Drying, Storage, and Shipping—
Important factors regarding storage of herbal products and how they should be maintained include the following.
- Light: Protection from light is important for botanical articles. Light accelerates numerous chemical processes that may lead to degradation or changes in the constituents of the articles.
- Temperature: Storage temperatures in this Pharmacopeia are defined in the General Notices. Excessive heat may affect the content of volatile constituents (essential oils) and accelerate degradation processes. However, heat treatments are sometimes useful in the maintenance of the article's quality and can be used in drying, reducing microbial load, and inhibiting certain enzymes. Heat application during these processes must be carefully controlled to achieve the desired balance between degradation and quality conservation.
- Humidity: Moisture in the articles may allow certain enzymes such as glycosidases to become active, hence degrading constituents. High humidity also increases the danger of microbial proliferation. As a rule, it is advisable to store botanical articles below 60% relative humidity. Although controlled humidity and temperature warehouses are now required in many good manufacturing practices for natural products, much of the world still lacks access to these facilities.
- Degree of Comminution: The degree of comminution plays a role in determining the stability of the botanical articles during storage. The increased surface area in fine powders allows oxidation and other degradation processes to occur more extensively and rapidly than in the case of a whole article. Plants containing tannins, bitter substances, and essential oils are particularly sensitive to the degree of comminution. In general, dried crude botanicals should be stored in a minimally processed form.
- Containers: Appropriate containers are defined in this Pharmacopeia in the General Notices.
Constituents—
Where known, the substances mainly responsible for the activity of the product are listed, along with other compounds contained in the plant.
Supplemental Information and General Guidance Protocols
Black Cohosh Actaea racemosa L. [Cimicifuga racemosa (L.) Nutt.] (Fam. Ranunculaceae)
Actaea racemosa L. [Cimicifuga racemosa (L.) Nutt.] (Fam. Ranunculaceae)
Botanical Identification—Actaea racemosa L.
Herbaceous perennial from rhizome.
Stem:
Erect, solitary, to 2.5 m tall, glabrous.
Leaves:
Basal and cauline, alternate, 2-4-ternately compound, petioles 15 to 60 cm long, bases clasping stem; leaflets 20 to 70; terminal leaflet of central division 3-lobed, 6 to 15 cm long, 6 to 16.5 cm wide, with 3 prominent veins arising from base; subterminal leaflets with blades ovate-lanceolate to obovate, 4 to 12 cm long and 3 to 8 cm wide; margins toothed to deeply incised; green above, paler below; glabrous or rarely pubescent along veins of undersurface.
Inflorescence:
Terminal panicle of 4 to 9 slender branches, each 7 to 60 cm long, pubescent; 1 bract subtending each pedicel.
Flowers:
Perfect, radially symmetric; sepals 4, greenish-white, caducous; petals 0; staminodes (1-) 4 (-8), petaloid, cream-colored, 2 to 3 mm long, clawed, apex bifid; stamens 55 to 110; pistils 1 (-3), glabrous to pubescent, ovary superior, style short, stigma 0.5 mm wide.
Fruit:
Many-seeded follicle, 5 to 10 mm long, ovoid, laterally compressed with curved, stout beak (peristent style), pubescent; seeds hemispheric, brown, scales lacking.
Chromosome number:
n = 8.
There are currently two varieties of A. racemosa recognized based on differences in leaf morphology: var. racemosa and var. dissecta. The former variety has triternate-pinnate leaves with serrate margins, while the latter has quadriternate-pinnate leaves that are deeply incised with serrate lobes. Variety dissecta is only known from very few herbarium specimens, all of which were collected well over 100 years ago, making this taxon of uncertain taxonomic significance.
Compendial History—
Black cohosh appeared on the secondary list of substances in the first United States Pharmacopeia (USP) of 1820, where it was listed as an anti-inflammatory and antispasmodic. It soon rose to the primary list in 1830, a position it held until the 10th decennial revision of 1920. Black cohosh appeared in the first edition of the United States Dispensatory (USD) in 1833 and remained through 1955 for a total of 122 years. Carrying forward the traditional Native American use of black cohosh for women's ailments and Barton's use for throat complaints, current therapeutics finds the plant used in a number of preparations for coughs and for gynecological disorders. In 2001, both the rhizome and the dry rhizome extract of black cohosh were proposed once again for inclusion in the United States Pharmacopeia–National Formulary (USP–NF). (See revised proposal on page 1455 of PF 28(5) [Sept.–Oct. 2002].) The monograph became official in the Second Supplement to USP 30–NF 25.
Constituents—
Major constituents of black cohosh are triterpene glycosides principally as beta-xylopyranosides and alpha-arabinopyranosides. The aglycones are mostly derived from acteol and cimigenol. The nomenclature of these compounds is quite confusing in the literature with different names often given to the same compounds. A cyclopropane ring is a common feature of these compounds, which are structurally related to cycloartenol. The isoflavone formononetin has been reported in some publications, however recent evidence indicates its absence in the roots and rhizomes of Actaea racemosa. Other constituents include tannins, resin, fatty acids, starch, sugars, and aromatic acids including ferulic acid, isoferulic acid, caffeic acid, and salicylic acid.
Sources—
Black cohosh can be found in moist deciduous forests, ravines, moist meadows, creek margins, and mountainous terrain. Black cohosh flowers from June to September and is native to eastern North America from Ontario south to Georgia and west to Missouri. The entire supply of black cohosh comes from the United States. The major producers of black cohosh are Kentucky and Tennessee, with additional supplies coming from Georgia, Ohio, North Carolina, Michigan, South Carolina, Virginia, West Virginia, and Wisconsin. Although there are reports of black cohosh being grown in China and India for export, the true identity of the cultivated material has not been verified and may well be an Asian species of Actaea such as A. cimicifuga (syn. Cimicifuga foetida). The vast majority of the commercial black cohosh is wild harvested. Concern over the conservation of black cohosh due to increasing demand makes this species a good candidate for cultivation.
Collection and Cultivation—
Collection (Conservation and Ecology)—
Traditionally, black cohosh has been harvested after plants become reproductive, which occurs anywhere from 2 to 8 years of age in cultivated plants depending on growing techniques (see Cultivation). A portion of the rhizome with a visible bud on it should be left in the ground to resprout the following year. There is no published information on the relationship between the constituent profile of the rhizome and its age, growing conditions, or place of origin, although such studies are underway. The impact of harvest on wild populations of black cohosh is currently unknown and sources differ in their opinion about it. While some maintain that current levels of harvest threaten the viability of wild populations, others feel that sustainable harvesting is possible at current levels of demand. A study of sustainable harvest limits is currently underway. The regulatory status regarding the trade of black cohosh is under review by the CITES. Refraining from harvesting plants until after they have set seed and leaving a portion of the rhizome in the ground to resprout are key components to sustainable harvesting.
Cultivation Practices—
Black cohosh is grown from rhizome cuttings or seeds and requires some shading, depending on altitude and other environmental conditions. If grown from rhizome cuttings, a plant takes 2 to 3 years to become reproductive; grown from seed sown in the greenhouse and then planted, takes 4 to 6 years; direct-seeded may take from 6 to 8 years. Preliminary work indicates that black cohosh can be propagated successfully using in vitro techniques.
Optimal Times for Harvest—
Rhizomes and roots should be harvested in autumn when the plant is dormant. At that time the underground portions of the plant have lower moisture content than in other seasons. Fall harvesting also allows plants to produce mature seeds before being uprooted.
Optimal Handling and Processing Practices—
Rhizomes with roots may be processed fresh or dried. They should be thoroughly washed directly after harvest and then laid out to dry. Freshly harvested roots should be solid but not woody.
Drying, Storage, and Shipping—
Drying—
Rhizomes with roots are cut and air-dried at 35 to 45
to 45 . They are fully dried when they are brittle and snap easily and when no moisture is evident in cross section, either visibly or to the touch.
. They are fully dried when they are brittle and snap easily and when no moisture is evident in cross section, either visibly or to the touch.
Storage—
Follow general guidelines for storage by packing in airtight containers protected from light, heat, moisture, and insect infestation.
Adulterants—
Other species of Actaea, especially yellow cohosh (A. podocarpa syn. Cimicifuga americana), have commonly been mixed with A. racemosa due to the similarity in above-ground appearance and common growing habitat between species. The two species can be distinguished by differences in their freshly harvested underground parts: the fresh rhizome of A. podocarpa has a distinct yellowish hue, whereas that of A. racemosa is black. The rhizomes of both species are far more difficult to tell apart when dry because A. podocarpa darkens upon drying. The underground portions of baneberry (Actaea pachypoda and A. rubra) occur as occasional adulterants of black cohosh supplies. Fruiting plants of baneberry may be distinguished from black cohosh by their fleshy white or red poisonous berries, which contrast with the dry follicles of black cohosh. No information was available on how to distinguish the underground portions of black cohosh and baneberry from each other. According to one herb dealer, the roots of baneberry are smaller than those of black cohosh, and therefore are not often harvested by wildcrafters. In the Pacific Northwest, Actaea elata (syn. Cimicifuga elata) is collected for medicinal use.
Ginger Zingiber officinale Roscoe (Fam. Zingiberaceae)
Zingiber officinale Roscoe (Fam. Zingiberaceae)
Botanical Identification—
Zingiber officinale Roscoe. Herbaceous perennial from tuberous rhizome, aromatic due to the presence of volatile oils.
Stem:
Erect, unbranched pseudostem formed by the tight overlap of sheathing leaf bases; 0.9 to 1.5 m tall.
Leaf:
Simple, alternate and two-ranked, sessile or petioles short with bases sheathing the stem and a ligule where the leaf base meets the stem; blade linear to narrowly lanceolate, 15 to 25 cm long, 1.5 to 3 cm wide; margin entire; glabrous to pubescent.
Inflorescence:
Terminal spike, 3.5 to 8 cm long, 1.5 to 2 cm wide, with conspicuous spirally arranged primary bracts; usually borne on specialized leafless stems.
Flower:
Perfect, bilaterally symmetric; calyx tubular with 3 lobes; corolla tube 2 to 2.5 cm long with lanceolate apical lobes, 1.5 to 2 cm long, 2 to 3.5 mm wide, greenish yellow; stamen 1, anther cream-colored with dark purple, elongated connective grasping upper part of style; staminodes 4, petaloid, 2 fused into an erect, ovate-oblong lip which is dull purple with cream mottling; ovary inferior; style 1, slender, exerted beyond connective.
Fruit:
Loculicidal capsule; seeds shiny black with a white aril.
Chromosome number:
n = 11.
There are several different varieties and forms of ginger. The varying morphological characteristics of these are displayed in Table 1.
Table 1. Morphological and Key Characteristics of Ginger from Different Areas of Production
| Source | Form | Aroma | Color (External) |
| Africa | Flat surfaces, mostly peeled, starchy and fibrous; 9 cm long, 1.5 cm wide |
Poor quality is recognized by its camphoraceous aroma | Uncut surface dark grayish- brown; cut surface brownish- black |
| Australia | Citrus-like | Buff | |
| Bengal | Flat surfaces, scraped | Gray-brown | |
| China | Short stumpy lobes, unscraped, mostly sliced |
Strong, floral to citrus | Pale brown |
| Cochin | Lateral surfaces lacking cork | Strong, floral to citrus | Cream color with numerous black resin dots |
| Jamaica (unbleached) |
Up to 12 cm long, 1 cm wide; surfaces completely peeled; starchy and fibrous thin cortex |
Delicate, citrus-like | All surfaces yellow-brown |
| Japan | Up to 7 cm long, 12 mm wide; flat surfaces usually completely peeled; starchy and fibrous thick cortex |
Bergamot-like | Externally gray-white to light grayish-brown, often with white powder from being coated with lime |
| Malabar (Cochin and Calcutta) |
Cork layer completely removed, mostly treated with chalk |
Citrus-like | Almost white |
| Nigeria | Smaller in size than other varieties, rather less deeply scraped |
Delicate | Somewhat darker than other varieties |
Compendial History—
Ginger was official in the United States Pharmacopoeia from the first edition of 1820 through the fourteenth revision of 1950, often appearing in multiple preparations. It also appeared in all editions of the United States Dispensatory from 1833 through the final edition of 1973 where it was described as “a stimulant and carminative that has been used for treatment of dispepsia and flatulent colic”.
Constituents—
The essential oils and the pungent principles make up some of the major components of the rhizome of ginger: 4.0% to 10.0% of the rhizome consists of an oleoresin composed of nonvolatile, pungent principles (phenols such as gingerols and their related dehydration products, shogaols); nonpungent fats and their waxes. The essential oil (1% to 3%) contains sesquiterpenes and monoterpenes, mainly geranial and nerals. Generally, but not always sesquiterpenes predominate (30% to 70%) such as zingiberene, sesquiphellandrene and beta-bisabolene, which decompose on drying and storage. The nonvolatile pungent principles include the phenylalkanones, gingerols, and the phenylalkanonols, shogaols with varying chain lengths.
Sources—
Ginger is cultivated in most tropical and subtropical countries to greater or lesser degrees. The world production is estimated to be 100,000 tons. China and India are reported to be the primary areas of production. Approximately 5000 tons of ginger are imported into the United States. An estimated 80% of this comes from China. In China, Sichuan and Guizhou provinces reportedly produce the largest quantities and highest quality. It is also produced in Zhejian, Shandong, Hubei, Guangdong, and Shanxi provinces. Most of the dried ginger from China available in the United States has had the cortex scraped or rubbed off before it is dried. This gives it a whitish appearance. The freshly dug root is soaked overnight in water, scraped with a knife to remove the outer cortex, and then sun-dried. It has been reported that high arsenic levels in the soil of Changning County of Hunan Province, China has negatively affected ginger yields.
In India, ginger is grown on a large scale in the warm, moist regions of Madras and Cochin, and to a lesser extent in Bengal and the Punjab. Varieties grown in Bengal are reportedly the highest quality material in India. Other areas of production include Africa (Nigeria and Sierra Leone), Australia, Fiji, East Indies, Jamaica, and Hawaii. The morphological characteristics of ginger cultivated in these different areas are outlined in Table 1.
In older literature, Jamaican ginger is reported to be the highest quality and the most aromatic, though supplies are limited.
Collection and Cultivation—
Collection (Conservation and Ecology)—
When the stems wither and are white, the rhizomes are ready for collection. Usually ginger is harvested after 6 months of growth at the earliest, and sometimes not until as late as 20 months, or to obtain larger roots it is harvested in January or February of the second year of growth. In tropical and subtropical areas, roots are harvested as early as 4 months of growth as they tend to become fibrous and tough as they get older. As ginger matures it becomes more fibrous and stronger in flavor. Ginger harvest can be described in three stages:
- Ginger that has been harvested early is known as green ginger and is traded as fresh ginger. It is succulent and tender, mellow, and mildly aromatic with a floral or lemony aroma and mild flavor.
- Ginger harvested a few months later is more fibrous and drier and is collected for drying and may be sold as a full-flavored, pungent dried whole ginger.
- The last harvest is usually around 9 months and yields the strongest ginger, which is quite dry and also richest in pungent components. This ginger is dried and then ground into powder.
Cultivation Practices—
Ginger is a perennial herb that grows well at subtropical temperatures where the rainfall is at least 1.98 meters per year. The plant is sterile and is grown by vegetative means. Selected pieces of rhizome (“seed pieces” or “setts”), each bearing a bud, are planted in holes or trenches. Ideally the soil should be well-drained, rich clay loam. The growing conditions resemble those of potato cultivation. Mulching or manuring is necessary because the plant rapidly exhausts the soil of nutrients.
Ginger is susceptible to water logging and root rot. Preventive methods include using only the cleanest ginger for planting and washing it with fungicide before planting. A study growing ginger hydroponically yielded up to 125 tons per hectare in 6 to 7 months compared to 35 tons per hectare when grown in soil.
Optimal Times for Harvest:
typically in December or January.
Optimal Handling and Processing Practices—
After harvesting, the rhizome is cleaned and stripped of its stems and roots. Each area processes its ginger differently after harvest. This results in the different quality and commercial grades available on the market. Green ginger consists of the rhizomes sent to market without drying. Unscraped or partially scraped varieties are traded as coated or black ginger. These roots have been scalded with boiling water and dried quickly. When dry, black ginger breaks with a horny, blackish, somewhat diaphanous fracture, due to the pasty condition of the starch. White ginger is bleached usually by rubbing with chalk or lime to lighten its color and to prevent insect infestation. Preserved ginger consists of soft, yellowish-brown pieces obtained by steeping the fresh ginger in hot syrup and carefully bottling. It is soft, brown-yellow and translucent. When baked, ginger loses its pungency and acquires a bitter taste.
Drying, Storage, and Shipping—
Drying—
In general, after harvest, the fresh roots are washed and the whole dark outer skin, consisting of cork and a little underlying parenchyma, is scraped away. Scraping speeds up the drying time of the crude drug. However, excessive scraping can result in lower concentrations of essential oil that is lost with the discarded epidermal tissue. After scraping, the rhizomes are then laid out on clean floors and dried in the sun for 7 to 10 days. During this time they are occasionally turned and are piled up every night. If the fresh rhizomes are too fleshy or moist, drying will take longer and the product will end up looking shriveled. To obtain a whiter product, the ginger is moistened after 5 or 6 days and dried for another 2 days at which time it is ready for export. Dried ginger is more pungent and stronger in taste than fresh ginger.
Storage—
Store in a tightly closed container, protected from light and moisture, in a cool area. A study was done on ginger harvested after 8, 9.5, 11, or 12 months. Samples were stored at 10 to 15
to 15 and 45% to 55% relative humidity or 25
and 45% to 55% relative humidity or 25 to 30
to 30 and 75% relative humidity for 0, 4, or 8 weeks. Oil and oleoresin yields increased with the age of the ginger. Room temperature storage had adverse effects but refrigerated storage for up to 4 weeks had no affect on quality. When stored for extended periods of time, ground ginger loses its pungency.
and 75% relative humidity for 0, 4, or 8 weeks. Oil and oleoresin yields increased with the age of the ginger. Room temperature storage had adverse effects but refrigerated storage for up to 4 weeks had no affect on quality. When stored for extended periods of time, ground ginger loses its pungency.
Adulterants—
Because ginger is so characteristic, unintentional adulterants are rare. However, in East Asia sometimes the much larger Zedoary cassumer and Zedoary zerumbet along with Alpinia allughas are used and found in European commerce. They are easy to distinguish due to their characteristic aromas. Occasionally, Chinese sugar-candied "ginger" is prepared from Alpinia galangal.
In older literature, other herbs have reportedly been used as adulterants. These include various species of Curcuma, Capsicum, and Grains of Paradise (Amomum melegueta) added to exhausted material in order to enhance the color and pungency.
Ginger powder is sometimes adulterated with plant starches such as those from wheat middlings, potatoes, corn, barley, rice, legumes, acorns, flaxseed meal, mannihot, oil cakes from linseed, raps, mustard, almond meal, palm kernal or olives, hazelnut shells, and mineral additives. These may be easily identified microscopically. The extent of this type of adulteration in trade is unknown.
Exhausted material should be considered an adulterant.
Valerian Valeriana officinalis L. (Fam. Valerianaceae)
Valeriana officinalis L. (Fam. Valerianaceae)
Botanical Identification—
Valeriana officinalis L. Herbacious perennial, rhizomatous.
Stem:
Solitary, hollow, 15 to 150 cm.
Leaf:
Basal and cauline, opposite, oddly once pinnately lobed, lobes 11 to 21 lanceolate, entire or dentate, basal leaves petiolate, cauline leaves subsessile to clasping.
Inflorescence:
Compound cyme, terminal or axillary, many pale pink to white, strongly scented flowers.
Flower:
Calyx 5-lobed, lobes inconspicuous in flower, becoming elongate and pappus-like in fruit, corolla funnel-form, slightly saccate at the base, 5-lobed, tube 4 mm, lobes 1 mm, stamens 3, filaments attached to corolla tube alternate to corolla lobes, ovary inferior, tri-loculate, uni-ovulate, only 1 locule fertile, stigma tripartite.
Fruit:
Achene crowned by persistent calyx, lanceolate-oblong, 4.5 to 5 mm, hairy or glabrous. Populations of V. officinalis range in ploidy level from diploid to tetraploid or octaploid. British V. officinalis is usually octaploid, and central European supplies are tetraploid.
There are three subspecies of V. officinalis: ssp. officinalis, ssp. collina (Wallr.) Nyman, and ssp. sambucifolia (Mikan fil.) Celak. All three of these subspecies, as well as the other European species of valerian, V. repens Host, have been considered acceptable source material for medicinal preparations.
Macroscopic Identification—
Various chemotypes will have slightly different characteristics. When dried, the whole rhizome is up to 50 mm long and up to 30 mm in diameter, obconical to cylindrical, with an elongated or compressed base. It has a yellowish-brown to dark brown exterior with a circular stem and leaf scars. The rhizome contains numerous thick, light to dark brown rootlets that are located around a thin ligneous cord. The root is longitudinally wrinkled and approximately 100 mm long and 1 to 3 mm in diameter, almost cylindrical and almost the same color as the rhizome. In longitudinal section, the pith exhibits a central cavity transversed by septa. The stolons are 20 to 50 mm long, pale yellowish grey with prominent nodes separated by longitudinally striated internodes. It is commonly sliced in half for ease of cleaning. The rootlets, which contain the majority of the essential oil, are brittle and break in short, horny fractures and are whitish or yellowish internally. Aroma: when dried properly, V. officinalis L., s.l. has only a very faint characteristic, valeric acid-like aroma that becomes stronger as it ages. Improperly dried or old material possesses a strong and characteristic odor due to the enzymatic hydrolysis of esters of the valepotriates (isovaleric acid and hydroxyvaleric acid). Taste: mildly sweet and camphoraceous with a slightly bitter and spicy aftertaste.
Compendial History—
Valerian was official in the United States Pharmacopeia from the first edition of 1820 through the eleventh revison of 1930, often appearing in multiple preparations. At its peak from 1850 through 1880 it appeared six to seven times in different preparations. Valerian is among the top 30 most listed botanicals in the history of the USP. The root of valerian has been used as a sedative and spasmolytic in Europe since the 16th century.
Constituents—
Major constituents of valerian have been identified as sesquiterpenes of volatile oils and iridoids (epoxy-triesters) known as valepotriates. The total content of volatile oil varies widely within a single species and between different species. European Valeriana officinalis L. usually contains 0.1% to 2.8% volatile oil. The oil consists of mixtures of monoterpene and sesquiterpene derivatives. The amount of valepotriates present also varies widely between species and genera and even within a species, generally ranging from 0.5% to 1.2%. Valepotriates are particularly unstable; they decompose easily under the effect of moisture, temperatures above 40 (104
(104 F), or acidity (pH < 3).
F), or acidity (pH < 3).
Valerian also contains small amounts of aliphatic acids, alkaloids, amino acids, phenolic acids, flavonoids, free fatty acids, sugars, and salts. Valerian constituents that have possible sedative effects include acetoxyvalerenic acid, 1-acevaltrate, baldrinal, didrovaltrate, hydroxyvalerenic acid, kessane derivatives, valeranone, valerenal, valerenic acid, and valtrate.
Sources—
Valerian is found in damp or dry meadows, scrub, or woods in most of Europe, but rare in the south, and is cultivated and naturalized in North America. Valerian is cultivated in Britain, Belgium, Eastern Europe, France, Germany, Holland, Japan, the Netherlands, North America, and Russia. The majority of standardized extract products and crude cut and sifted material on the domestic market are prepared from European supplies. A large number of liquid extracts are prepared from domestically cultivated material. Many species other than V. officinalis are reported to be traded as medicinal valerian. These include V. edulis Nutt. ex Torr. & A. Gray, V. corneana Briq. k, V. stubendorfi Kreyer ex Kom., V. amurensis P. Smirn. ex Kom., V. hardwickii Wall., V. exaltata Mikan, and V. wallichi DC. syn. V. jatamansi Jones.* The most frequently used North American species include V. sitchensis Bong and V. edulis Nutt.* = V. edulis Nutt. ex Torr. & Gray ssp. procera. Other species reported to be used locally include V. arizonica Gray, V. capitata Pall ex Link., V. diocia L., and V. scouleri Rydb. Detailed chemical analyses of most American species are lacking. A limited number of assays of material cultivated in the Pacific Northwest show varying levels of essential oil ranging from 0.4% to 1.3%. Valerenic acid and valepotriates have been found to be present in fresh and dry samples of V. sitchensis Bong. V. sitchensis Bong exhibits a strong pungency when fresh. High quality material is reported to contain from 1.0% to 1.5% essential oil, 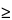 30% extractable matter, and
30% extractable matter, and 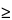 0.5% valerenic acid.
0.5% valerenic acid.
Collection and Cultivation—
Collection (Conservation and Ecology)—
The majority of valerian in trade comes from cultivated material. Harvest times will vary geographically. The composition of the essential oil varies greatly among different populations of the same subspecies and even between the same population of plants from year to year. Essential oil content also varies with genotypes, harvest times, growing conditions, age of root, drying techniques, and method of analysis. It has been reported that valerian harvested in higher elevations, grown in dryer regions, or cultivated in phosphate-rich soil yields relatively high levels of essential oil.
Older literature reports that valerian should be harvested in the fall, between August and September, preferably in the second year of growth. Analyses of material cultivated in the Netherlands report that the majority of constituents, including the essential oil and valerenic acid, were highest in roots harvested in the first year of growth with essential oil being highest in September and November (1.2% to 2.1%). The next highest level of essential oil was reported for material harvested in March (0.9% to 1.6%). Valerenic acid and its derivatives were found to be highest in February and March (0.7% to 0.9%) followed by material harvested in September (0.5 to 0.7%) and then in January (0.3% to 0.4%). From a commercial standpoint, it is more cost effective to harvest the roots in the same year the plants are sown than in the second year.
Cultivation Practices—
Sowing seeds has been reported to be preferred over planting of seedlings. Best results were achieved by flat field planting at row spacings of 50 cm and a seed rate of 3 kg per hectare. Cutting off the flowering tops before the plant has set seed causes the rhizome to develop more fully.
Optimal Times for Harvest—
Wagner reports that harvest should take place in the morning during relatively cool weather, a general recommendation for roots rich in essential oils.
Optimal Handling and Processing Practices—
The essential oil is located in the hypodermis of the rhizome in large thin-walled cells. Therefore, care must be taken not to damage these cells during handling. Excess washing of the roots can result in a significant reduction of extractive matter. Because of the sensitivity of volatile oils to heat, it is necessary to minimize the amount of time generated in the grinding or powdering process by doing small lots at a time, with frequent interruptions in run times, or by utilizing a cryogenic grinder.
Drying, Storage, and Shipping—
Drying—
For maximum preservation of the essential oils, valerian should be dried at 40 with a flow rate of 0.05 kg per sec per m2. Alternatively, drying at 20
with a flow rate of 0.05 kg per sec per m2. Alternatively, drying at 20 for approximately 10 days, shade drying at approximately 45
for approximately 10 days, shade drying at approximately 45 , low temperature vacuum-drying, and freeze-drying are also reported to be appropriate drying techniques.
, low temperature vacuum-drying, and freeze-drying are also reported to be appropriate drying techniques.
Careless or prolonged drying produces a darker color in the roots and results in the hydrolysis of the isovalerianic esters and the liberation of isovaleric and hydroxyisovaleric acid. This produces the characteristic valerianic aroma. Properly dried valerian will produce this same aroma over time.
Storage—
Store in closed containers protected from light, air, and moisture. Hydroxyvalerenic acid, a decomposition product of acetoxyvalerenic acid, is formed when the herb is stored at too high humidity.
Improper storage conditions can cause significant deterioration of the material. Although the essential oil is relatively stable, it can evaporate with excessive exposure to air. The essential oil can degrade quickly in powdered material. In powdered root, the essential oil content can decrease by 50% within 6 months.
Valepotriates are sensitive to humidity, temperatures above 40 , and acid media (pH <3) and are generally not detected in commercial products after 60 days.
, and acid media (pH <3) and are generally not detected in commercial products after 60 days.
Adulterants—
Other species of valerian: An unidentified Apiaceae species may be found in valerian trade. Adulteration of valerian in the American market is not common. Many species other than V. officinalis are reported to be traded as medicinal valerian. These include V. edulis Nutt. ex Torr. & A. Gray, V. coreana Briq.k, V. stubendorfi Kreyer ex Kom., V. amurensis P. Smirn. ex Kom., V. hardwickii Wall, V. exaltata Mikan, and V. wallichi DC. syn. V. jatamansi Jones.
*
V. wallichi DC. and V. edulis Nutt. reportedly are lacking in valerenic acid and its derivatives.
Auxiliary Information— Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| General Chapter | Yoshiyuki Tokiwa, Ph.D.
Senior Scientist 1-301-816-8321 |
(DSGC05) Dietary Supplements - General Chapters |
USP32–NF27 Page 777
Pharmacopeial Forum: Volume No. 33(5) Page 1029