Phase-solubility analysis is the quantitative determination of the purity of a substance through the application of precise solubility measurements. At a given temperature, a definite amount of a pure substance is soluble in a definite quantity of solvent. The resulting solution is saturated with respect to the particular substance, but the solution remains unsaturated with respect to other substances, even though such substances may be closely related in chemical structure and physical properties to the particular substance being tested. Constancy of solubility, like constancy of melting temperature or other physical properties, indicates that a material is pure or is free from foreign admixture except in the unique case in which the percentage composition of the substance under test is in direct ratio to solubilities of the respective components. Conversely, variability of solubility indicates the presence of an impurity or impurities.
Phase-solubility analysis is applicable to all species of compounds that are crystalline solids and that form stable solutions. It is not readily applicable to compounds that form solid solutions with impurities.
The standard solubility method consists of six distinct steps: (1) mixing, in a series of separate systems, increasing quantities of material with measured, fixed amounts of a solvent; (2) establishment of equilibrium for each system at identical constant temperature and pressure; (3) separation of the solid phase from the solutions; (4) determination of the concentration of the material dissolved in the various solutions; (5) plotting the concentration of the dissolved materials per unit of solvent (y-axis or solution composition) against the weight of material per unit of solvent (x-axis or system composition); and (6) extrapolation and calculation.
Solvents
A proper solvent for phase-solubility analysis meets the following criteria: (1) The solvent is of sufficient volatility that it can be evaporated under vacuum, but is not so volatile that difficulty is experienced in transferring and weighing the solvent and its solutions. Normally, solvents having boiling points between 60 and 150
and 150 are suitable. (2) The solvent does not adversely affect the substance being tested. Solvents that cause decomposition or react with the test substance are not to be used. Solvents that solvate or form salts are to be avoided, if possible. (3) The solvent is of known purity and composition. Carefully prepared mixed solvents are permissible. Trace impurities may affect solubility greatly. (4) A solubility of 10 mg to 20 mg per g is optimal, but a wider working range can be used.
are suitable. (2) The solvent does not adversely affect the substance being tested. Solvents that cause decomposition or react with the test substance are not to be used. Solvents that solvate or form salts are to be avoided, if possible. (3) The solvent is of known purity and composition. Carefully prepared mixed solvents are permissible. Trace impurities may affect solubility greatly. (4) A solubility of 10 mg to 20 mg per g is optimal, but a wider working range can be used.
Apparatus*
Constant-Temperature Bath—
Use a constant-temperature bath that is capable of maintaining the temperature within ±0.1 and that is equipped with a horizontal shaft capable of rotating at approximately 25 rpm. The shaft is equipped with clamps to hold the Ampuls. Alternatively, the bath may contain a suitable vibrator, capable of agitating the ampuls at 100 to 120 vibrations per second, and equipped with a shaft and suitable clamps to hold the ampuls.
and that is equipped with a horizontal shaft capable of rotating at approximately 25 rpm. The shaft is equipped with clamps to hold the Ampuls. Alternatively, the bath may contain a suitable vibrator, capable of agitating the ampuls at 100 to 120 vibrations per second, and equipped with a shaft and suitable clamps to hold the ampuls.
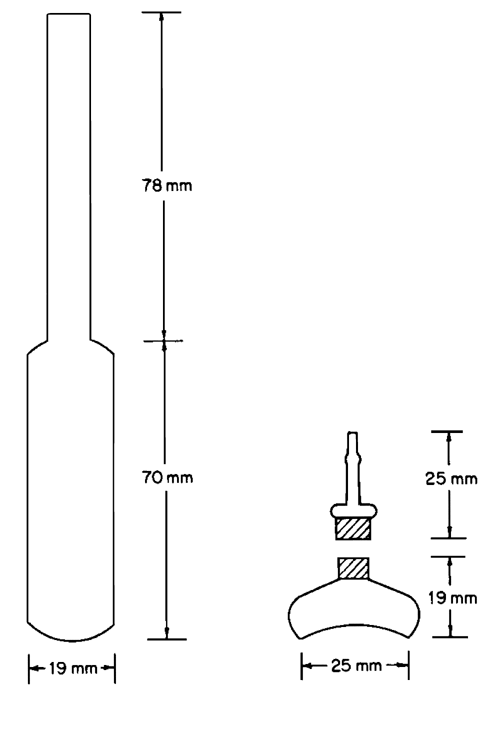
Ampuls—
Use 15-mL ampuls of the type shown in the accompanying illustration. Other containers may be used provided that they are leakproof and otherwise suitable.
Solubility Flasks—
Use solubility flasks of the type shown in the accompanying illustration.
Procedure
note—Make all weighings within ±10 µg.
System Composition—
Weigh accurately, in g, not less than 7 scrupulously cleaned 15-mL ampuls. Weigh accurately, in g, increasingly larger amounts of the test substance into each of the ampuls. The weight of the test substance is selected so that the first ampul contains slightly less material than will go into solution in 5 mL of the selected solvent, the second ampul contains slightly more material, and each subsequent ampul contains increasingly more material than meets the indicated solubility. Transfer 5.0 mL of the solvent to each of the ampuls, cool in a dry ice–acetone mixture, and seal, using a double-jet air-gas burner and taking care to save all glass. Allow the ampuls and their contents to come to room temperature, and weigh the individual sealed ampuls with the corresponding glass fragments. Calculate the system composition, in mg per g, for each ampul by the formula:
1000(W2 – W1) / (W3 – W2)
in which W2 is the weight of the ampul plus test substance, W1 is the weight of the empty ampul, and W3 is the weight of ampul plus test substance, solvent, and separated glass.
Equilibration—
The time required for equilibration varies with the substance, the method of mixing (rotation or vibration), and the temperature. Normally, equilibrium is obtained more rapidly by the vibration method (1 to 7 days) than by the rotational method (7 to 14 days). In order to determine whether equilibration has been effected, 1 ampul, i.e., the next to the last in the series, may be warmed to 40 to produce a supersaturated solution. Equilibration is ensured if the solubility obtained on the supersaturated solution falls in line with the test specimens that approach equilibrium from an undersaturated solution.
to produce a supersaturated solution. Equilibration is ensured if the solubility obtained on the supersaturated solution falls in line with the test specimens that approach equilibrium from an undersaturated solution.
Solution Composition—
After equilibration, place the ampuls vertically in a rack in the constant-temperature bath, with the necks of the ampuls above the water level, and allow the contents to settle. Open the ampuls, and remove a portion greater than 2 mL from each by means of a pipet equipped with a small pledget of cotton membrane or other suitable filter. Transfer a 2.0-mL aliquot of clear solution from each ampul to a marked, tared solubility flask, and weigh each flask plus its solution to obtain the weight of the solution. Cool the flasks in a dry ice–acetone bath, and then evaporate the solvent in vacuum. Gradually increase the temperature to a temperature consistent with the stability of the compound, and dry the residue to constant weight. Calculate the solution composition, in mg per g, by the formula:
1000(F3 – F1) / (F2 – F3)
in which F3 is the weight of the flask plus residue, F1 is the weight of the solubility flask, and F2 is the weight of the flask plus solution.
Calculation
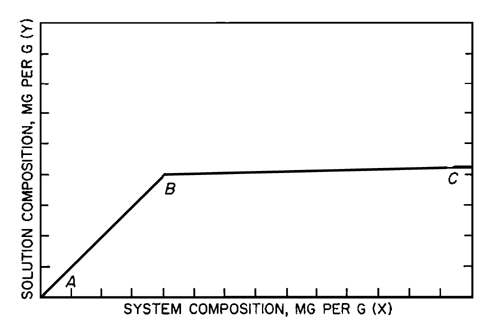
For each portion of the test substance taken, plot the solution composition as the ordinate and the system composition as the abscissa. As shown in the accompanying diagram,
the points for those containers, frequently only one, that represent a true solution fall on a straight line (AB) with a slope of 1, passing through the origin; the points corresponding to saturated solutions fall on another straight line (BC), the slope, S, of which represents the weight fraction of impurity or impurities present in the test substance. Failure of points to fall on a straight line indicates that equilibrium has not been achieved. A curve indicates that the material under test may be a solid solution. Calculate the percentage purity of the test substance by the formula:
100 – 100S.
The slope, S, may be calculated graphically or by least-squares treatment for best fit of the experimental values to a straight line.
The solubility of the main component is obtained by extending the solubility line (BC) through the y-axis. The point of interception on the y-axis is the extrapolated solubility, in mg per g, and is a constant for a given compound.
Purification Technique
Since the solvent phase in all combinations of solvent and solute that are used to construct segment BC of a phase-solubility diagram contains essentially all the impurities originally present in the substance under analysis, whereas the solid phase is essentially free from impurities, phase-solubility analysis can be used to prepare pure reference specimens of desired compounds as well as concentrates of impurities from substances otherwise considered pure. A simple modification of this technique can be used to accomplish these purposes with considerably less effort than is usually required for rigorous phase-solubility analysis.
In practice, a weighed amount of test specimen is suspended in a nonreactive solvent of suitable composition and amount so that about 10% of the material is dissolved at equilibrium. The suspension is sealed (a screw-cap vial is usually adequate) and shaken at room temperature until equilibrium is attained (usually 24 hours is sufficient for this purpose). The mother liquor is then drawn off and evaporated at or near room temperature to dryness. Since the mother liquor contained essentially all the impurities that were present in the specimen, the residue has been concentrated with respect to the impurities roughly in proportion to the ratio of the weight of specimen taken to the weight of solids dissolved in the volume of solvent used.
The undissolved crystals remaining after withdrawal of the mother liquor are usually sufficiently pure to be used as a reference standard after appropriate rinsing and drying.
*
Available from Hanson Research Corp., 19727 Bahama St., P. O. Box 35, Northridge, CA 91324.
Auxiliary Information— Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| General Chapter | Horacio N. Pappa, Ph.D.
Senior Scientist and Latin American Liaison 1-301-816-8319 |
(GC05) General Chapters 05 |
USP32–NF27 Page 687