- British Pharmacopoeia Volume I & II
- Monographs: Medicinal and Pharmaceutical Substances
Croscarmellose Sodium |
 |
(Ph. Eur. monograph 0985)
Excipient.
Ph Eur
Cross-linked sodium carboxymethylcellulose.
Sodium salt of a cross-linked, partly O-carboxymethylated cellulose.
White or greyish-white powder.
Practically insoluble in acetone, in anhydrous ethanol and in toluene.
A. Mix 1 g with 100 mL of a solution containing 4 ppm of methylene blue R, stir the mixture and allow it to settle. The substance to be examined absorbs the methylene blue and settles as a blue, fibrous mass.
B. Mix 1 g with 50 mL of water R. Transfer 1 mL of the mixture to a small test-tube and add 1 mL of water R and 0.05 mL of a freshly prepared 40 g/L solution of α-naphthol R in methanol R. Incline the test-tube and carefully add 2 mL of sulfuric acid R down the side so that it forms a lower layer. A reddish-violet colour develops at the interface.
C. The solution prepared from the sulfated ash in the test for heavy metals (see Tests) gives reaction (a) of sodium (2.3.1).
5.0 to 7.0 for the suspension.
Shake 1 g with 100 mL of carbon dioxide-free water R for 5 min.
Maximum 0.5 per cent (dried substance) for the sum of the percentage contents of sodium chloride and sodium glycollate.
Sodium chloride Place 5.00 g in a 250 mL conical flask, add 50 mL of water R and 5 mL of strong hydrogen peroxide solution R and heat on a water-bath for 20 min, stirring occasionally to ensure total hydration. Cool, add 100 mL of water R and 10 mL of nitric acid R. Titrate with 0.05 M silver nitrate, determining the end-point potentiometrically (2.2.20) using a silver indicator electrode and a double-junction reference electrode containing a 100 g/L solution of potassium nitrate R in the outer jacket and a standard filling solution in the inner jacket, and stirring constantly.
1 mL of 0.05 M silver nitrate is equivalent to 2.922 mg of NaCl.
Sodium glycollate Place a quantity of the substance to be examined equivalent to 0.500 g of the dried substance in a 100 mL beaker. Add 5 mL of glacial acetic acid R and 5 mL of water R and stir to ensure total hydration (about 15 min). Add 50 mL of acetone R and 1 g of sodium chloride R. Stir for several minutes to ensure complete precipitation of the carboxymethylcellulose. Filter through a fast filter paper impregnated with acetone R into a volumetric flask, rinse the beaker and the filter with 30 mL of acetone R and dilute the filtrate to 100.0 mL with the same solvent. Allow to stand for 24 h without shaking. Use the clear supernatant to prepare the test solution.
Prepare the reference solutions as follows: in a 100 mL volumetric flask, dissolve 0.100 g of glycollic acid R, previously dried in vacuo over diphosphorus pentoxide R at room temperature overnight, in water R and dilute to 100.0 mL with the same solvent; use the solution within 30 days; transfer 1.0 mL, 2.0 mL, 3.0 mL and 4.0 mL of the solution to separate volumetric flasks, dilute the contents of each flask to 5.0 mL with water R, add 5 mL of glacial acetic acid R, dilute to 100.0 mL with acetone R and mix.
Transfer 2.0 mL of the test solution and 2.0 mL of each of the reference solutions to separate 25 mL volumetric flasks. Heat the uncovered flasks for 20 min on a water-bath to eliminate acetone. Allow to cool and add 5.0 mL of 2,7-dihydroxynaphthalene solution R to each flask. Mix, add a further 15.0 mL of 2,7-dihydroxynaphthalene solution R and mix again. Close the flasks with aluminium foil and heat on a water-bath for 20 min. Cool and dilute to 25.0 mL with sulfuric acid R.
Measure the absorbance (2.2.25) of each solution at 540 nm. Prepare a blank using 2.0 mL of a solution containing 5 per cent V/V each of glacial acetic acid R and water R in acetone R. Prepare a standard curve using the absorbances obtained with the reference solutions. From the standard curve and the absorbance of the test solution, determine the mass (a) of glycollic acid in the substance to be examined, in milligrams, and calculate the content of sodium glycollate using the following expression:
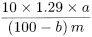
1.29 |
= |
the factor converting glycollic acid to sodium glycollate; |
b |
= |
loss on drying as a percentage; |
m |
= |
mass of the substance to be examined, in grams. |
Maximum 10.0 per cent.
Disperse 10.00 g in 800.0 mL of water R and stir for 1 min every 10 min during the first 30 min. Allow to stand for 1 h and centrifuge if necessary. Decant 200.0 mL of the supernatant liquid onto a fast filter paper in a vacuum filtration funnel, apply vacuum and collect 150.0 mL of the filtrate. Evaporate to dryness and dry the residue at 100-105 °C for 4 h.
Maximum 20 ppm.
To the residue obtained in the determination of the sulfated ash add 1 mL of hydrochloric acid R and evaporate on a water-bath. Take up the residue in 20 mL of water R. 12 mL of the solution complies with test A. Prepare the reference solution using lead standard solution (1 ppm Pb) R.
Maximum 10.0 per cent, determined on 1.000 g by drying in an oven at 105 °C for 6 h.
14.0 per cent to 28.0 per cent (dried substance), determined on 1.0 g, using a mixture of equal volumes of sulfuric acid R and water R.
TAMC: acceptance criterion 103 CFU/g (2.6.12).
TYMC: acceptance criterion 102 CFU/g (2.6.12).
Absence of Escherichia coli (2.6.13).
This section provides information on characteristics that are recognised as being relevant control parameters for one or more functions of the substance when used as an excipient ( see chapter 5.15 ). This section is a non-mandatory part of the monograph and it is not necessary to verify the characteristics to demonstrate compliance. Control of these characteristics can however contribute to the quality of a medicinal product by improving the consistency of the manufacturing process and the performance of the medicinal product during use. Where control methods are cited, they are recognised as being suitable for the purpose, but other methods can also be used. Wherever results for a particular characteristic are reported, the control method must be indicated.The following characteristics may be relevant for croscarmellose sodium used as disintegrant.
Place 75 mL of water R in a 100 mL graduated cylinder and add 1.5 g of the substance to be examined in 0.5 g portions, shaking vigorously after each addition. Dilute to 100.0 mL with water R and shake again until the substance is homogeneously distributed. Allow to stand for 4 h. The settling volume is between 10.0 mL and 30.0 mL.
0.60 to 0.85 (dried substance).
Place 1.000 g in a 500 mL conical flask, add 300 mL of a 100 g/L solution of sodium chloride R and 25.0 mL of 0.1 M sodium hydroxide, stopper the flask and allow to stand for 5 min, shaking occasionally. Add 0.05 mL of m-cresol purple solution R and about 15 mL of 0.1 M hydrochloric acid from a burette. Insert the stopper and shake. If the solution is violet, add 0.1 M hydrochloric acid in 1 mL portions until the solution becomes yellow, shaking after each addition. Titrate with 0.1 M sodium hydroxide until the colour turns to violet.
Calculate the number of milliequivalents (M) of base required to neutralise the equivalent of 1 g of dried substance.
Calculate the degree of acid carboxymethyl substitution (A) using the following expression:
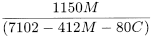
C |
= |
sulfated ash as a percentage. |
Calculate the degree of sodium carboxymethyl substitution (S) using the following expression:
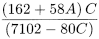
The degree of substitution is the sum of A and S.
Ph Eur

