- British Pharmacopoeia Volume I & II
- Monographs: Medicinal and Pharmaceutical Substances
Fluoxetine Hydrochloride |
 |
(Ph. Eur. monograph 1104)
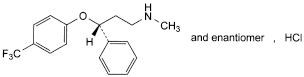
C17H18F3NO,HCl 345.8 59333-67-4
Selective serotonin reuptake inhibitor; antidepressant.
Ph Eur
(3RS)-N-Methyl-3-phenyl-3-[4-(trifluoromethyl)phenoxy]propan-1-amine hydrochloride.
98.0 per cent to 102.0 per cent (anhydrous substance).
White or almost white, crystalline powder.
Sparingly soluble in water, freely soluble in methanol, sparingly soluble in methylene chloride.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison fluoxetine hydrochloride CRS.
B. It gives reaction (a) of chlorides (2.3.1).
Dissolve 2.0 g in a mixture of 15 volumes of water R and 85 volumes of methanol R, then dilute to 100.0 mL with the same mixture of solvents.
Solution S is clear (2.2.1) and colourless (2.2.2, Method II).
4.5 to 6.5.
Dissolve 0.20 g in carbon dioxide-free water R and dilute to 20 mL with the same solvent.
- 0.05° to + 0.05°, determined on solution S.
Liquid chromatography (2.2.29).
Test solution (a) Dissolve 55 mg of the substance to be examined in the mobile phase and dilute to 10.0 mL with the mobile phase.
Test solution (b) Dilute 2.0 mL of test solution (a) to 10.0 mL with the mobile phase.
Reference solution Dissolve 22 mg of fluoxetine hydrochloride CRS in 10.0 mL of 0.5 M sulfuric acid. Heat at about 85 °C for 3 h. Allow to cool. The resulting solution contains considerable quantities of impurity A and usually also contains 4-trifluoromethylphenol. To 0.4 mL of this solution add 28.0 mg of fluoxetine hydrochloride CRS, about 1 mg of fluoxetine impurity B CRS and about 1 mg of fluoxetine impurity C CRS, then dilute to 25.0 mL with the mobile phase.
- — size: l = 0.25 m, Ø = 4.6 mm;
- — stationary phase: octylsilyl silica gel for chromatography R (5 µm).
Mobile phase Mix 8 volumes of methanol R, 30 volumes of tetrahydrofuran R and 62 volumes of a solution of triethylamine R prepared as follows: to 10 mL of triethylamine R, add 980 mL of water R, mix and adjust to pH 6.0 with phosphoric acid R (about 4.5 mL) and dilute to 1000 mL with water R.
Flow rate 1 mL/min.
Detection Spectrophotometer at 215 nm.
Injection 10 µL.
Run time 3 times the retention time of fluoxetine.
Identification of impurities Use the chromatogram obtained with the reference solution to identify the peaks due to impurities A, B and C.
Relative retention With reference to fluoxetine: impurity A = about 0.24; impurity B = about 0.27; impurity C = about 0.9
System suitability Reference solution:
- — retention time: fluoxetine = 10 min to 18 min; 4-trifluoromethylphenol: maximum 35 min; if no peak due to 4-trifluoromethylphenol is observed, inject a 0.02 per cent solution of 4-trifluoromethylphenol R in the mobile phase;
- — peak-to-valley ratio: minimum 11, where Hp = height above the baseline of the peak due to impurity C and Hv = height above the baseline of the lowest point of the curve separating this peak from the peak due to fluoxetine. If necessary, reduce the volume of methanol and increase the volume of the solution of triethylamine in the mobile phase.
Limit Test solution (b):
- — impurity C: not more than 0.0015 times the area of the principal peak (0.15 per cent).
Limits Test solution (a):
- — impurities A, B: for each impurity, not more than 0.0125 times the area of the principal peak in the chromatogram obtained with test solution (b) (0.25 per cent);
- — unspecified impurities: for each impurity, not more than 0.005 times the area of the principal peak in the chromatogram obtained with test solution (b) (0.10 per cent);
- — total: not more than 0.025 times the area of the principal peak in the chromatogram obtained with test solution (b) (0.5 per cent);
- — disregard limit: 0.0025 times the area of the principal peak in the chromatogram obtained with test solution (b) (0.05 per cent).
Gas chromatography (2.2.28).
Test solution Dissolve 50 mg of the substance to be examined in dimethylformamide R and dilute to 5.0 mL with the same solvent.
Reference solution To 1.0 g of acetonitrile R, add dimethylformamide R, mix and dilute to 100.0 mL with the same solvent. Dilute 1.0 mL of this solution to 1000.0 mL with dimethylformamide R.
- — material: fused silica;
- — size: l = 30 m, Ø = 0.53 mm;
- — stationary phase: macrogol 20 000 R (film thickness 1 µm).
Carrier gas helium for chromatography R.
Flow rate 10 mL/min.
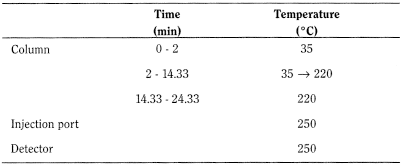
Temperature:
Detection Flame ionisation.
Injection 1 µL; inject dimethylformamide R as a blank.
In the chromatogram obtained with dimethylformamide R, verify that there is no peak with the same retention time as acetonitrile.
- — acetonitrile: not more than the area of the corresponding peak in the chromatogram obtained with the reference solution (0.1 per cent).
Maximum 20 ppm.
1.0 g complies with test C. Prepare the reference solution using 2 mL of lead standard solution (10 ppm Pb) R.
Maximum 0.5 per cent, determined on 1.00 g.
Maximum 0.1 per cent, determined on 1.0 g.
Liquid chromatography (2.2.29) as described in the test for related substances with the following modifications.
Test solution Dissolve 55.0 mg of the substance to be examined in the mobile phase and dilute to 50.0 mL with the mobile phase. Dilute 10.0 mL of this solution to 100.0 mL with the mobile phase.
Reference solution Dissolve 55.0 mg of fluoxetine hydrochloride CRS in the mobile phase and dilute to 50.0 mL with the mobile phase. Dilute 10.0 mL of this solution to 100.0 mL with the mobile phase.
Detection Spectrophotometer at 227 nm.
Retention time Fluoxetine = 10 min to 18 min; if necessary, adjust the volumes of methanol and of the solution of triethylamine in the mobile phase.
System suitability Reference solution:
- — symmetry factor: maximum 2.0 calculated at 10 per cent of the height of the peak due to fluoxetine.
Calculate the content of C17H19CIF3NO from the declared content of fluoxetine hydrochloride CRS.
Specified impurities A, B, C.
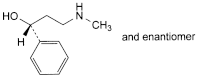
A. (1RS)-3-(methylamino)-1-phenylpropan-1-ol,
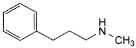
B. N-methyl-3-phenylpropan-1-amine,
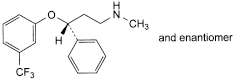
C. (3RS)-N-methyl-3-phenyl-3-[3-(trifluoromethyl)phenoxy]propan-1-amine.
Ph Eur

