- British Pharmacopoeia Volume III
- Formulated Preparations: Specific Monographs
Fluoxetine Oral Solution |
Selective serotonin reuptake inhibitor; antidepressant.
Fluoxetine Oral Solution is a solution of Fluoxetine Hydrochloride in a suitable flavoured vehicle.
The oral solution complies with the requirements stated under Oral Liquids and with the following requirements.
95.0 to 105.0% of the stated amount.
A. To a volume of the oral solution containing the equivalent of 0.1 g of fluoxetine add 5 mL of 4m sodium hydroxide, extract with 10 mL of dichloromethane, filter the dichloromethane layer (Whatman 1PS is suitable) and evaporate the filtrate to dryness. The infrared absorption spectrum of the residue, Appendix II A, is concordant with the reference spectrum of fluoxetine (RS 398).
B. In the Assay, the chromatogram obtained with solution (1) shows a peak with the same retention time as the principal peak in the chromatogram obtained with solution (2).
pH, 2.5 to 4.5, Appendix V L.
Carry out the method for liquid chromatography, Appendix III D, using the following solutions. Add 4.33 g of sodium octanesulfonate and 13.8 g of sodium dihydrogen orthophosphate monohydrate to 1000 mL of water, adjust the pH, if necessary, to 3.0 with orthophosphoric acid and filter (solution A). For solution (1) mix a quantity of the oral solution containing the equivalent of 20 mg of fluoxetine in 10 mL of a mixture of 1 volume of acetonitrile, 3 volumes of methanol and 6 volumes of solution A and use immediately. For solution (2) dilute 1 volume of solution (1) to 25 volumes with a mixture of 1 volume of acetonitrile, 3 volumes of methanol and 6 volumes of solution A and further dilute 1 volume to 10 volumes with the same solvent mixture. For solution (3) heat a 0.2% w/v solution of fluoxetine hydrochloride BPCRS in 0.5m sulfuric acid at 85° for 1 hour (generation of impurity A and chloro-impurity). For solution (4) dilute 1 volume of solution (3) to 100 volumes with a solution containing 0.1% w/v of fluoxetine hydrochloride BPCRS in a mixture of 1 volume of acetonitrile, 3 volumes of methanol and 6 volumes of solution A. Solution (5) contains 0.05% w/v of benzoic acid in a mixture of 1 volume of acetonitrile, 3 volumes of methanol and 6 volumes of solution A.
The chromatographic procedure may be carried out using (a) a stainless steel column (25 cm × 4.6 mm) packed with octadecylsilyl silica gel for chromatography (5 µm) (Supelco Supelcosil LC-18-DB is suitable), (b) as mobile phases A and B with a flow rate of 1 mL per minute the solutions described below and (c) a detection wavelength of 215 nm.
Mobile phase A Add 21 volumes of acetonitrile and 26 volumes of methanol to 53 volumes of solution A and mix.
Mobile phase B Add 35 volumes of acetonitrile and 22 volumes of methanol to 43 volumes of solution A and mix.
Inject 20 µL of each solution and record the chromatograms under the following conditions. Equilibrate the column with mobile phase A for at least 10 minutes. Elute initially with mobile phase A. After 13 minutes, use linear gradient elution increasing the concentration of mobile phase B to 100% after 2 minutes. Elute isocratically with mobile phase B for 14 minutes. Carry out a linear gradient elution for 1 minute decreasing the concentration of mobile phase B to 0% and elute for at least a further 10 minutes with mobile phase A.
When the chromatograms are recorded under the conditions described above the retention time of fluoxetine is 20 to 30 minutes.
The test is not valid unless, in the chromatogram obtained with solution (4), the peaks which elute before fluoxetine elute before the start of the gradient programme and are well separated from any peaks due to excipients. If necessary, adjust the proportion of acetonitrile in mobile phase A.
In the chromatogram obtained with solution (1), the area of any secondary peak is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.4%) and the sum of the areas of any such peaks is not greater than twice the area of the principal peak in the chromatogram obtained with solution (2) (0.8%). Disregard any peak with the same retention time as that of the principal peak in the chromatogram obtained with solution (5).
Carry out the method for liquid chromatography, Appendix III D, using the following solutions. For solution (1) dilute a quantity of the oral solution with sufficient of the mobile phase to produce a solution containing the equivalent of 0.004% w/v of fluoxetine. Solution (2) contains 0.0045% w/v of fluoxetine hydrochloride BPCRS in the mobile phase.
The chromatographic procedure may be carried out using (a) a stainless steel column (25 cm × 4.6 mm) packed with particles of silica, the surface of which has been modified with chemically bonded cyano groups, (5 µm) (Dupont Zorbax CN Special is suitable), (b) as the mobile phase with a flow rate of 1 mL per minute a mixture of equal volumes of a 1% v/v solution of triethylamine, adjusted to pH 6.0 with orthophosphoric acid, and acetonitrile and (c) a detection wavelength of 215 nm. For solution (1) allow the chromatography to proceed for at least 10 minutes.
Determine the weight per mL of the oral solution, Appendix V G, and calculate the content of C17H18F3NO, weight in volume, using the declared content of C17H18F3NO,HCl in fluoxetine hydrochloride BPCRS and taking each mg of C17H18F3NO,HCl to be equivalent to 0.8944 mg of C17H18F3NO.
Fluoxetine oral solution should be protected from light.
The quantity of active ingredient is stated in terms of the equivalent amount of fluoxetine.
In addition to impurities A and B listed under Fluoxetine Hydrochloride, the impurities limited by the requirements of this monograph include:
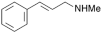
1. 3-Methylamino-1-phenylprop-1-ene,

2. 3-Methylamino-1-chloropropane,
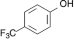
3. 4-Trifluoromethylphenol.

