- British Pharmacopoeia Volume I & II
- Monographs: Medicinal and Pharmaceutical Substances
Povidone |
 |
(Ph Eur monograph 0685)
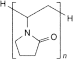
C6nH9n+2NnOn 9003-39-8
Disinfectant.
Ph Eur
α-Hydro-ω-hydropoly[1-(2-oxopyrrolidin-1-yl)ethylene]. It consists of linear polymers of 1-ethenylpyrrolidin-2-one.
11.5 per cent to 12.8 per cent of nitrogen (N; Ar 14.01) (anhydrous substance).
The different types of povidone are characterised by their viscosity in solution expressed as a K-value.
White or yellowish-white, hygroscopic powder or flakes.
Freely soluble in water, in ethanol (96 per cent) and in methanol, very slightly soluble in acetone.
First identification A, E.
Second identification B, C, D, E.
A. Infrared absorption spectrophotometry (2.2.24).
Preparation Dry the substances beforehand at 105 °C for 6 h; record the spectra using 4 mg of substance.
Comparison povidone CRS.
B. To 0.4 mL of solution S1 (see Tests) add 10 mL of water R, 5 mL of dilute hydrochloric acid R and 2 mL of potassium dichromate solution R. An orange-yellow precipitate is formed.
C. To 1 mL of solution S1 add 0.2 mL of dimethylaminobenzaldehyde solution R1 and 0.1 mL of sulfuric acid R. A pink colour is produced.
D. To 0.1 mL of solution S1 add 5 mL of water R and 0.2 mL of 0.05 M iodine. A red colour is produced.
E. To 0.5 g add 10 mL of water R and shake. The substance dissolves.
Dissolve 1.0 g in carbon dioxide-free water R and dilute to 20.0 mL with the same solvent. Add the substance to be examined to the water in small portions, stirring using a magnetic stirrer.
Dissolve 2.5 g in carbon dioxide-free water R and dilute to 25 mL with the same solvent. Add the substance to be examined to the water in small portions, stirring using a magnetic stirrer.
Solution S is clear (2.2.1) and not more intensely coloured than reference solution B6, BY6 or R6 (2.2.2, Method II).
3.0 to 5.0 for solution S, for povidone having a stated K-value of not more than 30; 4.0 to 7.0 for solution S, for povidone having a stated K-value of more than 30.
For povidone having a stated value of 18 or less, use a 50 g/L solution. For povidone having a stated value of more than 18 and not more than 95, use a 10 g/L solution. For povidone having a stated value of more than 95, use a 1.0 g/L solution. Allow to stand for 1 h and determine the viscosity (2.2.9) of the solution at 25 °C, using a size no. 1 viscometer with a minimum flow time of 100 s. Calculate the K-value using the following expression:
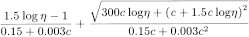
c |
= |
concentration of the substance to be examined, calculated with reference to the anhydrous substance, in grams per 100 mL; |
η |
= |
kinematic viscosity of the solution relative to that of water R. |
The K-value of povidone having a stated K-value of 15 or less is 85.0 per cent to 115.0 per cent of the stated value.
The K-value of povidone having a stated K-value or a stated K-value range with an average of more than 15 is 90.0 per cent to 108.0 per cent of the stated value or of the average of the stated range.
Maximum 500 ppm, expressed as acetaldehyde.
Test solution Dissolve 1.0 g of the substance to be examined in phosphate buffer solution pH 9.0 R and dilute to 100.0 mL with the same solvent. Stopper the flask tightly and heat at 60 °C for 1 h. Allow to cool to room temperature.
Reference solution Dissolve 0.140 g of acetaldehyde ammonia trimer trihydrate R in water R and dilute to 200.0 mL with the same solvent. Dilute 1.0 mL of the solution to 100.0 mL with phosphate buffer solution pH 9.0 R.
Into 3 identical spectrophotometric cells with a path length of 1 cm, introduce separately 0.5 mL of the test solution, 0.5 mL of the reference solution and 0.5 mL of water R (blank). To each cell add 2.5 mL of phosphate buffer solution pH 9.0 R and 0.2 mL of nicotinamide-adenine dinucleotide solution R. Mix and stopper tightly. Allow to stand at 22 ± 2 °C for 2-3 min and measure the absorbance (2.2.25) of each solution at 340 nm, using water R as the compensation liquid. To each cell add 0.05 mL of aldehyde dehydrogenase solution R, mix and stopper tightly. Allow to stand at 22 ± 2 °C for 5 min. Measure the absorbance of each solution at 340 nm using water R as the compensation liquid.
Calculate the content of aldehydes using the following expression:
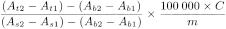
A t 1 |
= |
absorbance of the test solution before the addition of aldehyde dehydrogenase; |
A t 2 |
= |
absorbance of the test solution after the addition of aldehyde dehydrogenase; |
A s 1 |
= |
absorbance of the reference solution before the addition of aldehyde dehydrogenase; |
A s 2 |
= |
absorbance of the reference solution after the addition of aldehyde dehydrogenase; |
A b 1 |
= |
absorbance of the blank before the addition of aldehyde dehydrogenase; |
A b 2 |
= |
absorbance of the blank after the addition of aldehyde dehydrogenase; |
m |
= |
mass of povidone calculated with reference to the anhydrous substance, in grams; |
C |
= |
concentration of acetaldehyde in the reference solution, calculated from the weight of the acetaldehyde ammonia trimer trihydrate with the factor 0.72, in milligrams per millilitre. |
Maximum 400 ppm, expressed as H2O2.
Dissolve a quantity of the substance to be examined equivalent to 4.0 g of the anhydrous substance in water R and dilute to 100.0 mL with the same solvent (stock solution). To 25.0 mL of the stock solution add 2.0 mL of titanium trichloride-sulfuric acid reagent R. Allow to stand for 30 min. The absorbance (2.2.25) of the solution, measured at 405 nm using a mixture of 25.0 mL of the stock solution and 2.0 mL of a 13 per cent V/V solution of sulfuric acid R as the compensation liquid, is not greater than 0.35.
Liquid chromatography (2.2.29).
Test solution Dissolve a quantity of the substance to be examined equivalent to 2.0 g of the anhydrous substance in water R and dilute to 100.0 mL with the same solvent (test stock solution). Transfer a suspension of strongly acidic ion-exchange resin R for column chromatography in water R to a column of about 0.8 cm in internal diameter to give a packing of about 20 mm in length and keep the strongly acidic ion-exchange resin layer constantly immersed in water R. Pour 5 mL of water R and adjust the flow rate so that the water drops at a rate of about 20 drops per minute. When the level of the water comes down to near the top of the strongly acidic ion-exchange resin layer, put the test stock solution into the column. After dropping 2 mL of the solution, collect 1.5 mL of the solution and use this solution as the test solution.
Reference solution Dissolve 0.100 g of anhydrous formic acid R in water R and dilute to 100.0 mL with the same solvent. Dilute 1.0 mL of the solution to 100.0 mL with water R.
- — size: l = 0.25-0.30 m, Ø = 4-8 mm;
- — stationary phase: strongly acidic ion-exchange resin R for column chromatography (5-10 µm);
- — temperature: 30 °C.
Mobile phase Dilute 5 mL of perchloric acid R to 1000 mL with water R.
Flow rate Adjusted so that the retention time of formic acid is about 11 min.
Detection Spectrophotometer at 210 nm.
Injection 50 µL.
System suitability Reference solution:
- — repeatability: maximum relative standard deviation of 2.0 per cent after 6 injections.
- — formic acid: not more than 10 times the area of the principal peak in the chromatogram obtained with the reference solution (0.5 per cent).
Thin-layer chromatography (2.2.27). Use freshly prepared solutions.
Test solution Dissolve a quantity of the substance to be examined equivalent to 2.5 g of the anhydrous substance in 25 mL of water R. Add 0.5 mL of a 50 g/L solution of salicylaldehyde R in methanol R, mix and heat in a water-bath at 60 °C for 15 min. Allow to cool, add 2.0 mL of toluene R, shake for 2 min and centrifuge. Use the upper layer of the mixture.
Reference solution Dissolve 90 mg of salicylaldehyde azine R in toluene R and dilute to 100 mL with the same solvent. Dilute 1 mL of the solution to 100 mL with toluene R.
Plate TLC silanised silica gel plate F254 R.
Mobile phase water R, methanol R (1:2 V/V).
Application 10 µL.
Development Over 3/4 of the plate.
Drying In air.
Detection Examine in ultraviolet light at 365 nm.
Retardation factor Salicylaldehyde azine = about 0.3.
- — hydrazine: any spot due to salicylaldehyde azine is not more intense than the spot in the chromatogram obtained with the reference solution (1 ppm).
Liquid chromatography (2.2.29).
Test solution Dissolve a quantity of the substance to be examined equivalent to 0.250 g of the anhydrous substance in the mobile phase and dilute to 10.0 mL with the mobile phase.
Reference solution (a) Dissolve 50.0 mg of 1-vinylpyrrolidin-2-one R (impurity A) in methanol R and dilute to 100.0 mL with the same solvent. Dilute 1.0 mL of the solution to 100.0 mL with methanol R. Dilute 5.0 mL of this solution to 100.0 mL with the mobile phase.
Reference solution (b) Dissolve 10 mg of 1-vinylpyrrolidin-2-one R and 0.5 g of vinyl acetate R in methanol R and dilute to 100.0 mL with the same solvent. Dilute 1.0 mL of the solution to 100.0 mL with the mobile phase.
- — size: l = 0.025 m, Ø = 4 mm;
- — stationary phase: octadecylsilyl silica gel for chromatography R (5 µm).
- — size: l = 0.25 m, Ø = 4 mm;
- — stationary phase: octadecylsilyl silica gel for chromatography R (5 µm);
- — temperature: 40 °C.
Mobile phase acetonitrile R, water R (10:90 V/V).
Flow rate Adjusted so that the retention time of impurity A is about 10 min.
Detection Spectrophotometer at 235 nm.
Injection 50 µL; after injection of the test solution, wait for about 2 min and wash the precolumn by passing the mobile phase through the column backwards for 30 min at the same flow rate as applied in the test.
- — resolution: minimum 2.0 between the peaks due to impurity A and vinyl acetate in the chromatogram obtained with reference solution (b);
- — repeatability: maximum relative standard deviation of 2.0 per cent after 6 injections of reference solution (a).
- — impurity A: not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (10 ppm).
Liquid chromatography (2.2.29).
Test solution Dissolve a quantity of the substance to be examined equivalent to 0.100 g of the anhydrous substance in water R and dilute to 50.0 mL with the same solvent.
Reference solution Dissolve 0.100 g of 2-pyrrolidone R (impurity B) in water R and dilute to 100.0 mL with the same solvent. Dilute 3.0 mL of the solution to 50.0 mL with water R.
- — size: l = 0.025 m, Ø = 3 mm;
- — stationary phase: end-capped octadecylsilyl silica gel for chromatography R (5 µm).
- — size: l = 0.25 m, Ø = 3 mm;
- — stationary phase: end-capped octadecylsilyl silica gel for chromatography R (5 µm);
- — temperature: 30 °C.
Mobile phase water R adjusted to pH 2.4 with phosphoric acid R.
Flow rate Adjusted so that the retention time of impurity B is about 11 min.
Detection Spectrophotometer at 205 nm.
Injection 50 µL; after each injection of the test solution, wash away the polymeric material of povidone from the precolumn by passing the mobile phase through the column backwards for about 30 min at the same flow rate as applied in the test.
System suitability Reference solution:
- — repeatability: maximum relative standard deviation of 2.0 per cent after 6 injections.
- — impurity B: not more than the area of the principal peak in the chromatogram obtained with the reference solution (3.0 per cent).
Maximum 10 ppm.
2.0 g complies with test D. Prepare the reference solution using 2.0 mL of lead standard solution (10 ppm Pb) R.
Maximum 5.0 per cent, determined on 0.500 g.
Maximum 0.1 per cent, determined on 1.0 g.
Place 0.100 g of the substance to be examined (m mg) in a combustion flask and add 5 g of a mixture of 1 g of copper sulfate R, 1 g of titanium dioxide R and 33 g of dipotassium sulfate R, and 3 glass beads. Wash any adhering particles from the neck into the flask with a small quantity of water R. Add 7 mL of sulfuric acid R, allowing it to run down the insides of the flask. Heat the flask gradually until the solution has a clear, yellowish-green colour, and the inside wall of the flask is free from any carbonised material, and then heat for a further 45 min. After cooling, add cautiously 20 mL of water R, and connect the flask to the distillation apparatus, which has been previously washed by passing steam through it. To the absorption flask add 30 mL of a 40 g/L solution of boric acid R, 3 drops of bromocresol green-methyl red solution R and sufficient water to immerse the lower end of the condenser tube. Add 30 mL of strong sodium hydroxide solution R through the funnel, rinse the funnel cautiously with 10 mL of water R, immediately close the clamp on the rubber tube, then start distillation with steam to obtain 80-100 mL of distillate. Remove the absorption flask from the lower end of the condenser tube, rinsing the end part with a small quantity of water R, and titrate the distillate with 0.025 M sulfuric acid until the colour of the solution changes from green through pale greyish blue to pale greyish reddish-purple. Carry out a blank determination.
1 mL of 0.025 M sulfuric acid is equivalent to 0.7004 mg of N.
In an airtight container.
The label indicates the nominal K-value.
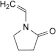
A. 1-ethenylpyrrolidin-2-one (1-vinylpyrrolidin-2-one),
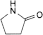
B. pyrrolidin-2-one (2-pyrrolidone).
This section provides information on characteristics that are recognised as being relevant control parameters for one or more functions of the substance when used as an excipient (see chapter 5.15). Some of the characteristics described in the Functionality-related characteristics section may also be present in the mandatory part of the monograph since they also represent mandatory quality criteria. In such cases, a cross-reference to the tests described in the mandatory part is included in the Functionality-related characteristics section. Control of the characteristics can contribute to the quality of a medicinal product by improving the consistency of the manufacturing process and the performance of the medicinal product during use. Where control methods are cited, they are recognised as being suitable for the purpose, but other methods can also be used. Wherever results for a particular characteristic are reported, the control method must be indicated.
The following characteristics may be relevant for povidone used as solubiliser and stabiliser in liquid dosage forms.
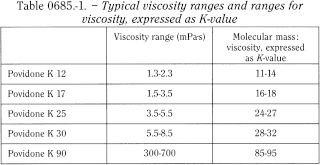
Determine the dynamic viscosity using a capillary viscometer on a 10 per cent solution (dried substance) at 25 °C. Typical values are shown in Table 0685.-1.
Typical values are shown in Table 0685.-1.
The following characteristic may be relevant for povidone used as binder in tablets and granules.
Typical values are shown in Table 0685.-1.
Ph Eur

