- British Pharmacopoeia (Veterinary)
- Monographs
- Medicinal and Pharmaceutical Substances
Cefalonium |
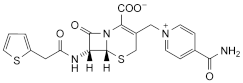
C20H18N4O5S2,2H2O 494.5 5575-21-3 (anhydrous)
Cephalosporin antibacterial.
Cefalonium is 3-(4-carbamoyl-1-pyridiniomethyl)-7-[(2-thienyl)acetamido]-3-cephem-4-carboxylate dihydrate. It contains not less than 95.0% and not more than 103.5% of C20H18N4O5S2, calculated with reference to the anhydrous substance.
A white or almost white crystalline powder.
Very slightly soluble in water and in methanol; soluble in dimethyl sulfoxide; insoluble in dichloromethane, in ethanol (96%) and in ether. It dissolves in dilute acids and in alkaline solutions.
A. The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of cefalonium (RSV 09).
B. The light absorption, Appendix II B, in the range 220 to 350 nm of a 0.002% w/v solution in water exhibits two maxima, at 235 nm and at 262 nm. The absorbance at 235 nm is about 0.76 and at 262 nm is about 0.62.
Dissolve 0.25 g with the aid of gentle heat in sufficient dimethyl sulfoxide to produce 50 mL. Allow the solution to stand for 30 minutes before measurement of the optical rotation. The specific optical rotation of the resulting solution is -50 to -56, calculated with reference to the anhydrous substance, Appendix V F.
Carry out the method for thin-layer chromatography, Appendix III A, using the following solutions in 8.3m acetic acid.
(1) 2.5% w/v of the substance being examined.
(2) 0.05% w/v of the substance being examined.
(3) 0.025% w/v of the substance being examined.
(4) 0.005% w/v of the substance being examined.
(5) 0.05% w/v of each of cefalotin sodium EPCRS and isonicotinamide.
(a) Use as the coating silica gel F254.
(b) Use the mobile phase as described below.
(c) Apply 4 µL of each solution.
(d) Develop the plate to 12 cm.
(e) After removal of the plate, allow it to dry in air and examine under ultraviolet light (254 nm).
10 volumes of glacial acetic acid, 10 volumes of 1m sodium acetate and 30 volumes of propan-2-ol.
The test is not valid unless the chromatogram obtained with solution (5) shows two clearly separated spots.
Any secondary spot in the chromatogram obtained with solution (1) is not more intense than the spot in the chromatogram obtained with solution (2) (2%), not more than one such spot is more intense than the spot in the chromatogram obtained with solution (3) (1%) and not more than three such spots are more intense than the spot in the chromatogram obtained with solution (4) (0.2% each).
Not more than 0.2%, Appendix IX A.
6.5 to 8.5% w/w, Appendix IX C. Use 0.5 g.
Measure the absorbance of a 0.002% w/v solution at the maximum at 262 nm, Appendix II B. Calculate the content of C20H18N4O5S2 from the absorbance obtained using a 0.002% w/v solution of cefalonium BPCRS and from the declared content of C20H18N4O5S2 in cefalonium BPCRS.
Cefalonium should be protected from light and stored at a temperature not exceeding 30°.
Cefalonium intended for use in the manufacture of either a parenteral dosage form or an intramammary infusion without a further appropriate sterilisation procedure complies with the following additional requirement.
Complies with the test for sterility, Appendix XVI A.
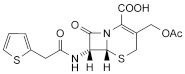
A. cefalotin,
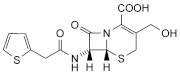
B. 3-hydroxymethyl-7β-(2-thienylacetamido)-3-cephem-4-carboxylic acid,
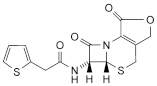
C. 3-hydroxymethyl-7β-(2-thienylacetamido)-3-cephem-4-carboxylic acid lactone,
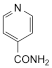
D. isonicotinamide.

