- British Pharmacopoeia Volume I & II
- Monographs: Medicinal and Pharmaceutical Substances
Flupentixol Hydrochloride |
 |
(Flupentixol Dihydrochloride, Ph Eur monograph 1693)
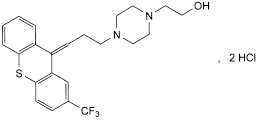
C23H25F3N2OS,2HCl 507.4 2413-38-9
Dopamine receptor antagonist; neuroleptic.
Ph Eur
2-[4-[3-[(EZ)-2-(trifluoromethyl)-9H-thioxanthen-9-ylidene]propyl]piperazin-1-yl]ethanol dihydrochloride.
- — flupentixol dihydrochloride: 98.0 per cent to 101.5 per cent (dried substance),
- — Z-isomer: 42.0 per cent to 52.0 per cent.
White or almost white powder.
Very soluble in water, soluble in alcohol, practically insoluble in methylene chloride.
First identification A, D.
Second identification B, C, D.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison flupentixol dihydrochloride CRS .
B. Thin-layer chromatography (2.2.27).
Test solution Dissolve 20 mg of the substance to be examined in methanol R and dilute to 10 mL with the same solvent.
Reference solution Dissolve 20 mg of flupentixol dihydrochloride CRS in methanol R and dilute to 10 mL with the same solvent.
Plate TLC silica gel F254 plate R.
Mobile phase water R, diethylamine R, methyl ethyl ketone R (1:4:95 V/V/V).
Application 2 µL.
Development Twice over a path of 15 cm.
Drying In air.
Detection A Examine in ultraviolet light at 254 nm.
Results A The principal spot in the chromatogram obtained with the test solution is similar in position and size to the principal spot in the chromatogram obtained with the reference solution. Doubling of the spot may be observed in both chromatograms.
Detection B Spray with alcoholic solution of sulfuric acid R; heat at 110 °C for 5 min and allow to cool; examine in ultraviolet light at 365 nm.
Results B The principal spot in the chromatogram obtained with the test solution is similar in position and size to the principal spot in the chromatogram obtained with the reference solution. Doubling of the spot may be observed in both chromatograms.
C. Mix about 5 mg with 45 mg of heavy magnesium oxide R and ignite in a crucible until an almost white residue is obtained (usually less than 5 min). Allow to cool, add 1 mL of water R, 0.05 mL of phenolphthalein solution R1 and about 1 mL of dilute hydrochloric acid R to render the solution colourless. Filter and add to the filtrate a freshly prepared mixture of 0.1 mL of alizarin S solution R and 0.1 mL of zirconyl nitrate solution R. Mix, allow to stand for 5 min and compare the colour of the solution with that of a blank prepared in the same manner. The test solution is yellow. The blank is red.
D. It gives reaction (a) of chlorides (2.3.1).
The solution is clear (2.2.1) and not more intensely coloured than reference solution GY6 (2.2.2, Method II).
Dissolve 2.0 g of the substance to be examined in water R and dilute to 20 mL with the same solvent.
2.0 to 3.0.
Dissolve 0.5 g in carbon dioxide-free water R and dilute to 50 mL with the same solvent.
Thin-layer chromatography (2.2.27). Carry out the test protected from light and prepare the solutions immediately before use.
Test solution (a) Dissolve 0.40 g of the substance to be examined in alcohol R and dilute to 20 mL with the same solvent.
Test solution (b) Dilute 2.0 mL of test solution (a) to 20.0 mL with alcohol R.
Reference solution (a) Dilute 1.0 mL of test solution (b) to 50.0 mL with alcohol R.
Reference solution (b) Dilute 2.0 mL of reference solution (a) to 20.0 mL with alcohol R.
Reference solution (c) Dissolve 10 mg of flupentixol impurity D CRS in alcohol R, add 0.5 mL of test solution (a) and dilute to 20.0 mL with alcohol R.
Plate TLC silica gel F254 plate R.
Mobile phase diethylamine R, toluene R, ethyl acetate R (10:20:70 V/V/V).
Application 5 µL.
Development In an unsaturated tank over a path of 10 cm.
Drying In air.
Detection Spray with alcoholic solution of sulfuric acid R, heat at 110 °C for 5 min and allow to cool; examine in ultraviolet light at 365 nm. Doubling of the spot due to flupentixol may be observed.
System suitability The chromatogram obtained with reference solution (c) shows 2 clearly separated spots.
- — in the chromatogram obtained with test solution (a): any spots, apart from the principal spot, are not more intense than the spot, or spots in the chromatogram obtained with reference solution (a) (0.2 per cent),
- — in the chromatogram obtained with test solution (b): any spots, apart from the principal spot, are not more intense than the spot or spots in the chromatogram obtained with reference solution (b) (0.2 per cent).
Liquid chromatography (2.2.29).Carry out the test protected from light and prepare the solutions immediately before use.
Test solution Dissolve 20.0 mg of the substance to be examined in the mobile phase and dilute to 20.0 mL with the mobile phase.
Reference solution Dissolve 10.0 mg of flupentixol dihydrochloride CRS and 10.0 mg of flupentixol impurity F CRS in the mobile phase and dilute to 100.0 mL with the mobile phase. Dilute 1.0 mL of the solution to 20.0 mL with the mobile phase.
- — size: l = 0.125 m, Ø = 4.6 mm,
- — stationary phase: octylsilyl silica gel for chromatography R (3 µm)
Mobile phase Mix 10 volumes of acetonitrile R, 55 volumes of methanol R and 35 volumes of a solution containing 8.72 g/L of potassium dihydrogen phosphate R, 0.37 g/L of anhydrous disodium hydrogen phosphate R and 0.77 g/L of dodecyltrimethylammonium bromide R.
Flow rate 1.0 mL/min.
Detection Spectrophotometer at 270 nm.
Injection 20 µL.
System suitability Reference solution:
- — resolution: minimum 2.0 between the 2nd of the peaks due to impurity F and the 1st of the peaks due to flupentixol. Peak splitting may not always occur.
- — impurity F: not more than the area of the corresponding peak or peaks in the chromatogram obtained with the reference solution (0.5 per cent).
Maximum 20 ppm.
1.0 g complies with limit test C. Prepare the standard using 2 mL of lead standard solution (10 ppm Pb) R.
Maximum 2.0 per cent, determined on 1.000 g by drying in an oven at 105 °C.
Maximum 0.1 per cent, determined on 1.0 g in a platinum crucible.
Dissolve 0.200 g in 30 mL of alcohol R. Carry out a potentiometric titration (2.2.20), using 0.1 M sodium hydroxide. Read the volume added between the 2 points of inflexion.
1 mL of 0.1 M sodium hydroxide is equivalent to 50.74 mg of C23H27Cl2F3N2OS.
Liquid chromatography (2.2.29).
Test solution Dissolve 20.0 mg of the substance to be examined in the mobile phase and dilute to 50.0 mL with the mobile phase.
Reference solution Dissolve 20.0 mg of flupentixol dihydrochloride CRS in the mobile phase and dilute to 50.0 mL with the mobile phase.
- — size: l = 0.25 m, Ø = 4.0 mm,
- — stationary phase: silica gel for chromatography R (5 µm).
Mobile phase water R, concentrated ammonia R, 2-propanol R, heptane R (2:4:150:850 V/V/V/V).
Flow rate 1.5 mL/min.
Detection Spectrophotometer at 254 nm.
Injection 20 µL.
System suitability Reference solution:
- — resolution: minimum 3.0 between the peaks due to Z-isomer (1st peak) and to E-isomer (2nd peak).
- — calculate the percentage content of Z-isomer taking into account the assigned content of Z-isomer in flupentixol dihydrochloride CRS,
- — calculate the ratio of the area of the peak due to the E-isomer to the area of the peak due to the Z-isomer: this ratio is 0.9 to 1.4.
Protected from light.
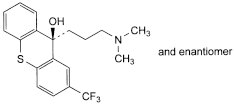
A. (9RS)-9-[3-(dimethylamino)propyl]-2-(trifluoromethyl)-9H-thioxanthen-9-ol,
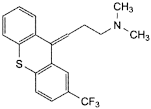
B. N,N-dimethyl-3-[(EZ)-2-(trifluoromethyl)-9H-thioxanthen-9-ylidene]propan-1-amine,
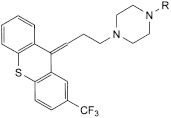
C. R = H: 1-[3-[(EZ)-2-(trifluoromethyl)-9H-thioxanthen-9-ylidene]propyl]piperazine,
D. R = CH2-CH2-O-CH2-CH2-OH: 2-[2-[4-[3-[(EZ)-2-(trifluoromethyl)-9H-thioxanthen-9-ylidene]propyl]piperazin-1-yl]ethoxy]ethanol,
E. R = CH2-CH2-O-CO-CH3: 2-[4-[3-[(EZ)-2-(trifluoromethyl)-9H-thioxanthen-9-ylidene]propyl]piperazin-1-yl]ethyl acetate,
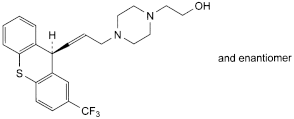
F. 2-[4-[(EZ)-3-[(9RS)-2-(trifluoromethyl)-9H-thioxanthen-9-yl]prop-2-enyl]piperazin-1-yl]ethanol,
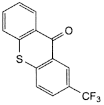
G. 2-(trifluoromethyl)-9H-thioxanthen-9-one.
Ph Eur