- British Pharmacopoeia Volume III
- Formulated Preparations: Specific Monographs
Insulin Zinc Suspension (Crystalline) |
 |
(Insulin Zinc Injectable Suspension (Crystalline), Ph Eur monograph 0836)
Hormone; treatment of diabetes mellitus.
Ph Eur
Insulin zinc injectable suspension (crystalline) complies with the monograph on Insulin preparations, injectable (0854) with the amendments prescribed below.
Insulin zinc injectable suspension (crystalline) is a sterile neutral suspension of bovine, porcine or human insulin, complexed with a suitable zinc salt; the insulin is in a form which is practically insoluble in water.
A white or almost white suspension which on standing deposits a white or almost white sediment and leaves a colourless or almost colourless supernatant liquid; the sediment is readily resuspended by gently shaking. When examined under a microscope, the particles are seen to be rhombohedral crystals, the majority having a maximum dimension when measured from corner to corner through the crystal greater than 10 µm but rarely exceeding 40 µm.
Examine the chromatograms obtained in the Assay. The position of the peak due to insulin in the chromatogram obtained with the test solution corresponds to that of the principal peak in the chromatogram obtained with the appropriate reference solution.
Not less than 90 per cent of the total insulin content. Centrifuge a volume of the substance to be examined containing 200 IU of insulin and discard the supernatant liquid. Suspend the residue in 1.65 mL of water R, add 3.3 mL of buffered acetone solution R, stir for 3 min, again centrifuge, discard the supernatant liquid and repeat all the operations with the residue. Dissolve the residue using a suitable procedure, for example dissolve in 0.1 M hydrochloric acid to give a final volume of 2.0 mL. Determine the insulin content of the residue (R) and determine the total insulin content (T) of an equal volume of the suspension by a suitable method. Calculate the percentage of insulin not extractable with buffered acetone solution from the expression:
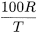
0.12 mg to 0.25 mg per 100 IU of insulin, determined as described in the monograph on Insulin preparations, injectable (0854).
20 per cent to 65 per cent of the total zinc is in the form of zinc in solution. Determine by the method described in the monograph on Insulin preparations, injectable (0854).
Ph Eur