Psyllium Hemicellulose
» Psyllium Hemicellulose is the alkali soluble fraction of the husk from Plantago ovata Forssk. It consists of a combination of highly substituted arabinoxylan polysaccharides. These polysaccharides are linear chains of xylose units (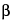 -(1®4)-xylan) to which are attached single units of arabinose and additional xylose. Rhamnose, galactose, glucose, and rhamnosyluronic acid residues are also present as minor constituents. It contains not less than 75.0 percent of dietary soluble fiber, calculated on the dried basis.
-(1®4)-xylan) to which are attached single units of arabinose and additional xylose. Rhamnose, galactose, glucose, and rhamnosyluronic acid residues are also present as minor constituents. It contains not less than 75.0 percent of dietary soluble fiber, calculated on the dried basis.
Packaging and storage—
Preserve in tight containers. Store at 25 , excursions permitted between 15
, excursions permitted between 15 and 30
and 30 .
.
Identification—
A:
The powdered mucilage stains red with ruthenium red TS and lead acetate TS.
B:
It meets the requirements of the test for Swell volume.
Total acidity—
To a beaker, transfer 40 mL of the supernatant as obtained below in the test for Swell volume without disturbing the gel. Add 1 mL of phenolphthalein TS, and titrate with 0.03 N sodium hydroxide. Not more than 1.8 mL is consumed.
Microbial enumeration tests  61
61 and Tests for specified microorganisms
and Tests for specified microorganisms  62
62 —
The total aerobic microbial count does not exceed 1000 cfu per g and the total combined molds and yeasts count does not exceed 100 cfu per g. It meets the requirements of the tests for absence of Salmonella species and Escherichia coli.
—
The total aerobic microbial count does not exceed 1000 cfu per g and the total combined molds and yeasts count does not exceed 100 cfu per g. It meets the requirements of the tests for absence of Salmonella species and Escherichia coli.
Loss on drying  731
731 —
Dry at 105
—
Dry at 105 for 3 hours: it loses not more than 12.0% of its weight.
for 3 hours: it loses not more than 12.0% of its weight.
Total ash  561
561 :
not more than 5.0%.
:
not more than 5.0%.
Acid-insoluble ash  561
561 :
not more than 1.0%.
:
not more than 1.0%.
Limit of alcohol—
Internal standard solution—
Transfer 5.0 mL of n-propyl alcohol into a 500-mL volumetric flask containing approximately 450 mL of water. Dilute with water to volume, insert the stopper into the flask, and mix well.
Standard stock solution—
Transfer 5.0 mL of absolute alcohol at 20 ± 2 into a 500-mL volumetric flask containing approximately 450 mL of water. Dilute with water to volume, insert the stopper into the flask, and mix well.
into a 500-mL volumetric flask containing approximately 450 mL of water. Dilute with water to volume, insert the stopper into the flask, and mix well.
Standard solution—
Transfer 10.0 mL of the Standard stock solution and 10.0 mL of Internal standard solution into a 100-mL volumetric flask. Dilute with water to volume, insert the stopper into the flask, and mix well.
Test solution—
Transfer 0.5 g of Psyllium Hemicellulose, accurately weighed, into a 150-mL conical flask. Add about 90 mL of water, insert the stopper into the flask, and stir rapidly for 3 hours using a magnetic stirrer. Add 10.0 mL of the Internal standard solution, and mix well. Pass the sample through a filter having a 0.45-µm porosity.
Chromatographic system (see Chromatography  621
621 )—
The gas chromatograph is equipped with a flame-ionization detector and a 0.53-mm × 30-m fused silica analytical column coated with 3.0-µm G43 stationary phase. A 0.53-mm × 2-m fused silica guard column may be used. The chromatograph is programmed as follows. Initially, the column temperature is equilibrated at 40
)—
The gas chromatograph is equipped with a flame-ionization detector and a 0.53-mm × 30-m fused silica analytical column coated with 3.0-µm G43 stationary phase. A 0.53-mm × 2-m fused silica guard column may be used. The chromatograph is programmed as follows. Initially, the column temperature is equilibrated at 40 for 5 minutes. The temperature is then increased at a rate of 10
for 5 minutes. The temperature is then increased at a rate of 10 per minute to 230
per minute to 230 , and is maintained at 230
, and is maintained at 230 for 3 minutes. The injection port temperature is maintained at 250
for 3 minutes. The injection port temperature is maintained at 250 , and the detector is maintained at 300
, and the detector is maintained at 300 . The carrier gas is helium. The split flow ratio is about 10:1, and the flow rate is maintained at about 4.0 mL per minute. Inject the Standard solution, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 2%.
. The carrier gas is helium. The split flow ratio is about 10:1, and the flow rate is maintained at about 4.0 mL per minute. Inject the Standard solution, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 2%.
Procedure—
Separately inject equal volumes (about 0.5 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the responses for all the peaks. Calculate the percentage of alcohol in the portion of Psyllium Hemicellulose taken by the formula:
1000(C/W)(RU / RS)
in which C is the concentration, in mg per mL, of alcohol in the Standard stock solution; W is the weight, in mg, of Psyllium Hemicellulose taken; and RU and RS are the ratios of the peak responses of alcohol to those of n-propyl alcohol from the Test solution and the Standard solution, respectively: not more than 12.0% (w/w) is found.
Heavy metals, Method II  231
231 :
10 µg per g.
:
10 µg per g.
Swell volume—
Add 0.50 g of Psyllium Hemicellulose to a glass-stoppered, 100-mL graduated mixing cylinder. To avoid material clumping, hold the cylinder at a 45 angle, and gently rotate it while using a wash bottle to forcefully add about 30 mL of water. Add water to bring the total volume to 100 mL, and cap the cylinder. Invert the cylinder several times until a uniform suspension is achieved, and allow to stand. Gently invert the cylinder several times again at 4 hours and 8 hours after the initial sample preparation, and allow to stand. Allow the gel to settle for 16 hours. Determine the volume of the gel: not less than 80 mL per g of Psyllium Hemicellulose is found.
angle, and gently rotate it while using a wash bottle to forcefully add about 30 mL of water. Add water to bring the total volume to 100 mL, and cap the cylinder. Invert the cylinder several times until a uniform suspension is achieved, and allow to stand. Gently invert the cylinder several times again at 4 hours and 8 hours after the initial sample preparation, and allow to stand. Allow the gel to settle for 16 hours. Determine the volume of the gel: not less than 80 mL per g of Psyllium Hemicellulose is found.
Content of soluble dietary fiber—
Alcohol solution—
Transfer 82.0 mL of alcohol to a 100-mL volumetric flask, dilute with water to volume, and mix.
Buffer solution—
Dissolve 1.95 g of 2-(N-morpholino)-ethanesulfonic acid and 1.22 g of tris(hydroxymethyl)aminomethane in 170 mL of water. Adjust with 6 N sodium hydroxide to a pH of 8.2, dilute with water to 200 mL, and mix. [note—It is important to adjust the pH to 8.2 at 24 . If the Buffer solution temperature is 20
. If the Buffer solution temperature is 20 , adjust the pH to 8.3; if the temperature is 28
, adjust the pH to 8.3; if the temperature is 28 , adjust the pH to 8.1. For deviations between 20
, adjust the pH to 8.1. For deviations between 20 and 28
and 28 , adjust by interpolation.]
, adjust by interpolation.]
Acid solution—
Prepare 0.561 N hydrochloric acid by dissolving 9.35 mL of 6 N hydrochloric acid in 70 mL of water. Dilute with water to 100.0 mL, and mix.
Phosphate buffer—
Prepare a pH 6.0 phosphate buffer (see Buffer Solutions under Reagents, Indicators, and Solutions).
Protease solution—
Dissolve 5 mg of protease in 0.1 mL of Phosphate buffer.
Enzyme purity—
To ensure the absence of undesirable enzymatic activities and the presence of desirable enzymatic activities, proceed as directed for Test preparations and Procedure using the substrates listed in the following table in place of Psyllium Hemicellulose.
The enzyme preparation is suitable if more than 90% of the original weight of pectin, arabinogalactan, and 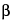 -glucan is recovered; not more than 2% of the original weight of casein and corn starch is recovered; and not more than 1% of the original weight of wheat starch is recovered. [note—Test the enzyme purity of every new lot of enzyme and at 6-month intervals thereafter.]
-glucan is recovered; not more than 2% of the original weight of casein and corn starch is recovered; and not more than 1% of the original weight of wheat starch is recovered. [note—Test the enzyme purity of every new lot of enzyme and at 6-month intervals thereafter.]
| Substrate | Weight in g | Activity Tested |
| Pectin | 0.2 | Pectinase |
| Arabinogalactan | 0.2 | Hemicellulase |
| 0.2 | ||
| Wheat starch | 1.0 | |
| Corn starch | 1.0 | |
| Casein | 0.3 | Protease |
Blank preparations—
Using two 400-mL tall-form beakers, appropriately labeled, proceed as directed for Procedure without Psyllium Hemicellulose.
Test preparations—
Weigh accurately, in duplicate, approximately 0.2 g of Psyllium Hemicellulose, previously milled to very fine powder. [note—Duplicates should differ by less than 1 mg in weight.] Transfer duplicate samples to appropriately labeled 400-mL, tall-form beakers, and proceed as directed for Procedure.
Procedure—
Treat each preparation in the following manner. Add 40 mL of Buffer solution to the beaker. [note—For the Test preparation, stir until Psyllium Hemicellulose is completely dispersed.] Add 125 µL of heat-stable 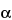 -amylase solution, and stir to ensure uniform mixing. Cover the beaker with aluminum foil, and incubate over a water bath maintained at 95
-amylase solution, and stir to ensure uniform mixing. Cover the beaker with aluminum foil, and incubate over a water bath maintained at 95 to 100
to 100 for 15 minutes, with continuous agitation. [note—Start timing once the water bath temperature reaches 95
for 15 minutes, with continuous agitation. [note—Start timing once the water bath temperature reaches 95 ; a total time of 35 minutes is usually sufficient.] Remove the beaker from the water bath, and cool to 60
; a total time of 35 minutes is usually sufficient.] Remove the beaker from the water bath, and cool to 60 . Remove the aluminum foil, scrape any ring from inside the beaker, and disperse any gels in the bottom of the beaker with a spatula. Rinse the walls of the beaker and the spatula with 10 mL of water, collecting the rinsings in the beaker. Add 500 µL of Protease solution. Cover with aluminum foil, and incubate over a water bath maintained at 60 ± 3
. Remove the aluminum foil, scrape any ring from inside the beaker, and disperse any gels in the bottom of the beaker with a spatula. Rinse the walls of the beaker and the spatula with 10 mL of water, collecting the rinsings in the beaker. Add 500 µL of Protease solution. Cover with aluminum foil, and incubate over a water bath maintained at 60 ± 3 for 30 minutes with continuous agitation. [note—Start timing when the bath temperature reaches 60
for 30 minutes with continuous agitation. [note—Start timing when the bath temperature reaches 60 .] Remove the foil, and transfer 5 mL of Acid solution while stirring. Adjust, if necessary, with 1 N sodium hydroxide or 1 N hydrochloric acid to a pH of 4.28 ± 0.07 at 60
.] Remove the foil, and transfer 5 mL of Acid solution while stirring. Adjust, if necessary, with 1 N sodium hydroxide or 1 N hydrochloric acid to a pH of 4.28 ± 0.07 at 60 . [note—It is important to adjust the pH to 4.28 while the solution in the beaker is maintained at 60
. [note—It is important to adjust the pH to 4.28 while the solution in the beaker is maintained at 60 , otherwise the pH will increase at lower temperatures.] Add 150 µL of amyloglucosidase solution while stirring. Cover with aluminum foil, and incubate over a water bath maintained at 60 ± 3
, otherwise the pH will increase at lower temperatures.] Add 150 µL of amyloglucosidase solution while stirring. Cover with aluminum foil, and incubate over a water bath maintained at 60 ± 3 for 30 minutes with constant agitation. [note—Start timing once the water bath reaches 60
for 30 minutes with constant agitation. [note—Start timing once the water bath reaches 60 .] Transfer approximately 40 mL of the beaker contents to a 50-mL centrifuge tube, and sonicate the tube contents for 3 minutes.* Centrifuge at 10,000–14,000 rpm for 10 minutes. Carefully pour the supernatant into an appropriately labeled 600-mL tared beaker. Do not disturb any pellet in the bottom of the centrifuge tube. Add the remaining sample from the original 400-mL beaker into the centrifuge tube still containing the pellet. Rinse the 400-mL beaker with 15–20 mL of water, and add the rinsing to the 50-mL centrifuge tube. Centrifuge the sample at 10,000–14,000 rpm for 10 minutes. Carefully pour the supernatant into the 600-mL beaker containing the first supernatant. Add 390 mL (measured before heating) of alcohol at 60
.] Transfer approximately 40 mL of the beaker contents to a 50-mL centrifuge tube, and sonicate the tube contents for 3 minutes.* Centrifuge at 10,000–14,000 rpm for 10 minutes. Carefully pour the supernatant into an appropriately labeled 600-mL tared beaker. Do not disturb any pellet in the bottom of the centrifuge tube. Add the remaining sample from the original 400-mL beaker into the centrifuge tube still containing the pellet. Rinse the 400-mL beaker with 15–20 mL of water, and add the rinsing to the 50-mL centrifuge tube. Centrifuge the sample at 10,000–14,000 rpm for 10 minutes. Carefully pour the supernatant into the 600-mL beaker containing the first supernatant. Add 390 mL (measured before heating) of alcohol at 60 to the 600-mL beaker. Cover the beaker, and allow to stand for at least 1 hour to form a precipitate.
to the 600-mL beaker. Cover the beaker, and allow to stand for at least 1 hour to form a precipitate.
Place 3 g of chromatographic siliceous earth into a clean air-dried crucible with a fritted disk. Heat the crucible containing chromatographic siliceous earth at 525º in a muffle furnace for at least 4 hours. Cool. Pass deionized water through the crucible while applying constant suction. Rinse with acetone, and allow to air-dry. Store the crucible in a convection oven at approximately 130º for at least 2 hours before use. Weigh the prepared crucible to 0.1 mg before use. Wet the chromatographic siliceous earth in the crucible using a stream of Alcohol solution from a washing bottle, and apply suction to evenly distribute the chromatographic siliceous earth over the fritted disk. Maintaining the suction, transfer the supernatant and precipitate from the beaker to the crucible, and filter. Transfer any solid residue in the beaker with the aid of Alcohol solution. [note—In some cases, gums may form during filtration, trapping liquid in the residue. If so, break the surface film with a spatula to improve filtration.] Wash the residue in the crucible sequentially with 30 mL of Alcohol solution, 20 mL of alcohol, and 20 mL of acetone. Dry the crucible containing the residue at 100 in a convection oven for at least 4 hours, cool to room temperature in a desiccator.
in a convection oven for at least 4 hours, cool to room temperature in a desiccator.
Determine the weight of the residue (R).
Use one of the duplicate residues from the Test preparations and one of the blank residues from the Blank preparations to determine the protein content, in mg, by placing the residue in a 500-mL Kjeldahl flask, and proceeding as directed for Method I under Nitrogen Determination  461
461 . The protein content is determined by multiplying the content of nitrogen found by 6.25. Incinerate the residue from the remaining duplicate of the Test preparation and the Blank preparation as directed for Total Ash under Articles of Botanical Origin
. The protein content is determined by multiplying the content of nitrogen found by 6.25. Incinerate the residue from the remaining duplicate of the Test preparation and the Blank preparation as directed for Total Ash under Articles of Botanical Origin  561
561 at a reduced temperature of 525
at a reduced temperature of 525 , and determine the ash content as directed. Calculate the corrected average weight of the blank, in mg, B, by the formula:
, and determine the ash content as directed. Calculate the corrected average weight of the blank, in mg, B, by the formula:
RB – PB – AB
in which RB is the weight, in mg, of the average blank residue for duplicate blank determinations; PB is the content, in mg, of protein found in the blank; and AB is the content, in mg, of ash found in the blank. Calculate the content of soluble dietary fiber, in percentage, by the formula:
100(RU – PU – AU – B)/WU
in which RU is the weight, in mg, of average residue for the duplicate Test preparations; PU is the content of protein, in mg, found in the Psyllium Hemicellulose; AU is the content of ash, in mg, found in the Psyllium Hemicellulose; B is the average weight of the blank as calculated above; and WU is the average weight, in mg, of the Psyllium Hemicellulose taken.
*
A suitable sonicator is Sonifier 250 (or equivalent), equipped with a 12-mm tip, from Branson Ultrasonic Corp., Danbury, CT, in which an output control value of 3 and a cycle time of 75% generates a power output of 43 W.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| Monograph | Maged H. Sharaf, Ph.D.
Senior Scientist 1-301-816-8318 |
(DSB05) Dietary Supplements - Botanicals |
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance | |
| Radhakrishna S Tirumalai, Ph.D.
Senior Scientist 1-301-816-8339 |
(MSA05) Microbiology and Sterility Assurance |
USP32–NF27 Page 3443
Pharmacopeial Forum: Volume No. 30(1) Page 173