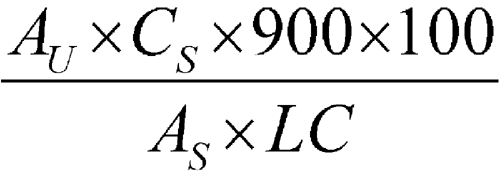Nefazodone Hydrochloride Tablets
» Nefazodone Hydrochloride Tablets contain not less than 90.0 percent and not more than 110.0 percent of the labeled amount of nefazodone hydrochloride (C25H32ClN5O2·HCl).
Packaging and storage—
Preserve in tight containers. Store at controlled room temperature.
USP Reference standards  11
11 —
—
USP Nefazodone Hydrochloride RS .
.
USP Nefazodone Related Compound A RS .
.
USP Nefazodone Related Compound B RS .
.
USP Nefazodone Hydrochloride RS
 .
.
USP Nefazodone Related Compound A RS
 .
.
USP Nefazodone Related Compound B RS
 .
.
Identification—
B:
The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
Dissolution  711
711 —
—
Medium:
0.1 N hydrochloric acid; 900 mL, deaerated.
Apparatus 2:
50 rpm.
Time:
30 minutes.
Standard stock solution—
Transfer about 70 mg of USP Nefazodone Hydrochloride RS, accurately weighed, to a 50-mL volumetric flask. Add 2.5 mL of methanol, dilute with Medium to volume, and mix.
Standard solution—
Dilute the Standard stock solution with Medium in such a way that the final concentration is similar to the one expected in the Test solution.
Test solution—
Use portions of the solution under test passed through a 0.45-µm PVDF filter, discarding the first 5 mL.
Procedure—
Determine the percentage of the labeled amount of nefazodone hydrochloride dissolved by employing UV absorption, using a suitable spectrophotometer, at the wavelength of maximum absorbance at about 246 nm, on the Test solution in comparison with the Standard solution, using Medium as the blank. Calculate the percentage of nefazodone hydrochloride (C25H32ClN5O2·HCl) dissolved by the formula:
in which AU and AS are the absorbances obtained from the Test solution and the Standard solution, respectively; CS is the concentration, in mg per mL, of USP Nefazodone Hydrochloride RS in the Standard solution; 900 is the volume, in mL, of Medium; 100 is the conversion factor to percentage; and LC is the tablet label claim, in mg.
Tolerances—
Not less than 75% (Q) of the labeled amount of C25H32ClN5O2·HCl is dissolved in 30 minutes.
Uniformity of dosage units  905
905 :
meet the requirements.
:
meet the requirements.
Related compounds—
Dilute acetic acid, Buffer solution, and Mobile phase—
Proceed as directed in the Assay.
Nefazodone related compound A stock solution—
Prepare a solution of USP Nefazodone Related Compound A RS in Mobile phase having a known concentration of about 80 µg per mL.
Nefazodone related compound B stock solution—
Prepare a solution of USP Nefazodone Related Compound B RS in Mobile phase having a known concentration of about 80 µg per mL.
System suitability solution—
Transfer about 10 mg of USP Nefazodone Hydrochloride RS into a 10-mL volumetric flask. Add 2.0 mL of Nefazodone related compound A stock solution and 2.0 mL of Nefazodone related compound B stock solution, and mix to dissolve the nefazodone hydrochloride. Dilute with Mobile phase to volume, and mix.
Standard solution—
Use the Standard preparation, prepared as directed in the Assay.
Test solution—
Use the Assay stock preparation, prepared as directed in the Assay.
Chromatographic system—
Prepare as directed in the Assay. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure. Identify the peaks using the relative retention times given in Table 1: the resolution, R, between nefazodone related compound A and nefazodone hydrochloride is not less than 2.0; and the resolution, R, between nefazodone related compound B and nefazodone hydrochloride is not less than 1.5. [note—Approximate relative retention times are provided in Table 1 for informational purposes only.]
Procedure—
Inject equal volumes (about 10 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the peak responses. Calculate the percentage of individual nefazodone related compounds in the portion of Tablets taken by the formula:
100(rU / rS)(CS / CT)(1/F)
in which rU is the individual peak response for each nefazodone related compound obtained from the Test solution; rS is the response of the corresponding peak in the Standard solution, respectively; CS and CT are the concentrations, in mg per mL, of nefazodone hydrochloride in the Standard solution and the Test solution, respectively; and F is the relative response factor obtained from Table 1. The related compound requirements are listed in Table 1.
Table 1
| Related Compound | Relative Retention Time |
Relative Response Factor (F) |
Limit (%) |
| Nefazodone related compound A | 1.4 | 1.2 | 0.2 |
| Nefazodone related compound B | 0.9 | 1.0 | 0.2 |
| Any individual unknown impurity | — | 1.0 | 0.2 each |
| Total known and unknown | — | — | 0.5 |
Assay—
Dilute acetic acid—
Prepare a mixture of acetic acid and water (1:1).
Buffer solution—
Dissolve 0.77 g of ammonium acetate in 1 L of water. Add 1.0 mL of triethylamine, and mix well. Adjust with Dilute acetic acid to a pH of 7.10 ± 0.05, and mix.
Mobile phase—
Prepare a filtered and degassed mixture of acetonitrile and Buffer solution (58:42). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard preparation—
Prepare a solution of USP Nefazodone Hydrochloride RS in Mobile phase having a known concentration of about 0.1 mg per mL.
Assay stock preparation—
Weigh and finely powder not fewer than 20 Tablets. Transfer an accurately weighed portion of the powder, equivalent to about 250 mg of nefazodone hydrochloride, based on the label claim, to a 250-mL volumetric flask, add about 125 mL of Mobile phase, and sonicate for about 10 minutes with occasional shaking. Dilute with Mobile phase to volume, and mix to obtain a solution having a concentration of about 1 mg per mL of nefazodone hydrochloride. Pass a portion of this solution through a filter having a 0.45-µm or finer porosity, and use the filtrate, which has a concentration of about 1 mg per mL of nefazodone hydrochloride.
Assay preparation—
Transfer 5.0 mL of Assay stock preparation into a 50-mL volumetric flask. Dilute with Mobile phase to volume, and mix to obtain a solution having a concentration of 0.1 mg per mL of nefazodone hydrochloride.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 250-nm detector and a 4.6-mm × 25-cm column containing 5-µm L1 packing. The flow rate is about 1.0 mL per minute. The column temperature is maintained at 30
)—
The liquid chromatograph is equipped with a 250-nm detector and a 4.6-mm × 25-cm column containing 5-µm L1 packing. The flow rate is about 1.0 mL per minute. The column temperature is maintained at 30 . Inject the Standard preparation, and record the peak responses as directed for Procedure: the tailing factor is not more than 2.0; and the relative standard deviation for replicate injections is not more than 2.0%.
. Inject the Standard preparation, and record the peak responses as directed for Procedure: the tailing factor is not more than 2.0; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in percent of label claim, of nefazodone hydrochloride (C25H32ClN5O2·HCl) in the portion of Tablets taken by the formula:
100(CS / CU)(rU / rS)
in which CS and CU are the concentrations, in mg per mL, of nefazodone hydrochloride in the Standard preparation and the Assay preparation, respectively; and rU and rS are the peak areas obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Ravi Ravichandran, Ph.D.
Senior Scientist 1-301-816-8330 |
(MDPP05) Monograph Development-Psychiatrics and Psychoactives |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
|
| Margareth R.C. Marques, Ph.D.
Senior Scientist 1-301-816-8106 |
(BPC05) Biopharmaceutics05 |
USP32–NF27 Page 3044
Pharmacopeial Forum: Volume No. 32(3) Page 804
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.