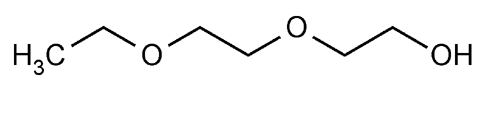
Diethylene Glycol Monoethyl Ether
» Diethylene Glycol Monoethyl Ether contains not less than 99.0 percent and not more than 101.0 percent of C6H14O3. It is produced by condensation of ethylene oxide and alcohol, followed by distillation.
Packaging and storage—
Preserve in tight containers under an atmosphere of an inert gas, at a temperature not exceeding 35 .
.
Labeling—
The label indicates that it is stored under an atmosphere of an inert gas.
Identification—
A:
Infrared Absorption  197F
197F , potassium bromide plates being used.
, potassium bromide plates being used.
B:
The retention time of the major peak in the chromatogram of the Assay preparation corresponds to that in the chromatogram of the Standard preparation, as obtained in the Assay.
Refractive index  831
831 :
between 1.426 and 1.428 at 20
:
between 1.426 and 1.428 at 20 .
.
Water, Method I  921
921 :
not more than 0.1%, determined on a 10-g specimen.
:
not more than 0.1%, determined on a 10-g specimen.
Acid value  401
401 —
[note—This test must be performed promptly after sampling to avoid oxidation of the test specimen.] Dissolve about 30 g, accurately weighed, in 30 mL of neutralized alcohol, add 1 mL of phenolphthalein TS, and titrate with 0.01 N alcoholic potassium hydroxide VS to produce a permanent, faint pink color: the acid value so obtained is not more than 0.1.
—
[note—This test must be performed promptly after sampling to avoid oxidation of the test specimen.] Dissolve about 30 g, accurately weighed, in 30 mL of neutralized alcohol, add 1 mL of phenolphthalein TS, and titrate with 0.01 N alcoholic potassium hydroxide VS to produce a permanent, faint pink color: the acid value so obtained is not more than 0.1.
Peroxide value  401
401 :
not more than 8.0, about 2 g, accurately weighed, being used.
:
not more than 8.0, about 2 g, accurately weighed, being used.
Limit of free ethylene oxide—
Acetaldehyde solution—
Prepare a solution of acetaldehyde in water containing a known concentration of about 10 µg per mL. [note—Prepare the Acetaldehyde solution fresh just prior to use.]
Ethylene oxide stock solution—
[Caution—Ethylene oxide is toxic and flammable. Prepare these solutions in a well-ventilated fume hood, using great care. Protect both hands and face by wearing polyethylene protective gloves and an appropriate face mask.
][note—Before using the polyethylene glycol 200 in this test, remove any volatile components from it by placing 500 mL of the polyethylene glycol 200 in a 1000-mL round bottom flask, attaching the flask to a rotary evaporator, and evaporating at a temperature of 60 and at a pressure of 1.5 kPa to 2.5 kPa for 6 hours.] Fill a chilled pressure bottle with liquid ethylene oxide, and store in a freezer when not in use. Use a small piece of polyethylene film to protect the liquid from contact with the rubber gasket. Tare a glass-stoppered conical flask, add about 50 mL of polyethylene glycol 200, and reweigh the flask. Transfer about 5 mL of the liquid ethylene oxide to a 100-mL beaker chilled in a mixture of sodium chloride and wet ice (1:3). Using a gas-tight gas chromatographic syringe that has been previously cooled to
and at a pressure of 1.5 kPa to 2.5 kPa for 6 hours.] Fill a chilled pressure bottle with liquid ethylene oxide, and store in a freezer when not in use. Use a small piece of polyethylene film to protect the liquid from contact with the rubber gasket. Tare a glass-stoppered conical flask, add about 50 mL of polyethylene glycol 200, and reweigh the flask. Transfer about 5 mL of the liquid ethylene oxide to a 100-mL beaker chilled in a mixture of sodium chloride and wet ice (1:3). Using a gas-tight gas chromatographic syringe that has been previously cooled to  10
10 , transfer about 300 µL (corresponding to about 250 mg) of liquid ethylene oxide to the polyethylene glycol 200, and swirl gently to mix. Replace the stopper, reweigh the flask, and determine the amount of ethylene oxide absorbed by weight difference. Adjust the weight of the mixture with polyethylene glycol 200 to 100.0 g, replace the stopper, and swirl gently to mix. This stock solution contains about 2.5 mg of ethylene oxide per g. [note—Prepare this stock solution fresh just prior to use, and store in a refrigerator.]
, transfer about 300 µL (corresponding to about 250 mg) of liquid ethylene oxide to the polyethylene glycol 200, and swirl gently to mix. Replace the stopper, reweigh the flask, and determine the amount of ethylene oxide absorbed by weight difference. Adjust the weight of the mixture with polyethylene glycol 200 to 100.0 g, replace the stopper, and swirl gently to mix. This stock solution contains about 2.5 mg of ethylene oxide per g. [note—Prepare this stock solution fresh just prior to use, and store in a refrigerator.]
Ethylene oxide standard solution A—
Tare a glass-stoppered conical flask, and chill it in a refrigerator. Add about 35 mL of polyethylene glycol 200, and reweigh the flask. Using a gas-tight gas chromatographic syringe that has been chilled in a refrigerator, transfer about 1 g of the chilled Ethylene oxide stock solution, accurately weighed, to the tared, conical flask. Adjust the weight of the solution with polyethylene glycol 200 to 50.0 g, replace the stopper, and swirl gently to mix. Transfer about 10 g of this solution, accurately weighed, to a 50-mL volumetric flask. Add 30 mL of water, and mix. Dilute with water to volume, and mix to obtain a solution containing about 10 µg of ethylene oxide per mL. [note—Prepare this solution fresh just prior to use, and store in a refrigerator.]
Ethylene oxide standard solution B—
Transfer 10.0 mL of Ethylene oxide standard solution A to a 50-mL volumetric flask, dilute with water to volume, and mix to obtain a solution containing about 2 µg of ethylene oxide per mL. [note—Prepare this solution fresh just prior to use, and store in a refrigerator.]
Standard solutions—
Transfer 0.5 mL of Ethylene oxide standard solution B to a 10-mL pressure headspace vial, add 0.1 mL of Acetaldehyde solution and 0.1 mL of water, seal the vial, and mix. Heat the mixture at 70 for 45 minutes to obtain Standard solution A. Transfer about 1 g of Diethylene Glycol Monoethyl Ether, accurately weighed, to a 10-mL pressure headspace vial, add 0.5 mL of Ethylene oxide standard solution B and 0.5 mL of water. Seal the vial, and mix. Heat the mixture at 70
for 45 minutes to obtain Standard solution A. Transfer about 1 g of Diethylene Glycol Monoethyl Ether, accurately weighed, to a 10-mL pressure headspace vial, add 0.5 mL of Ethylene oxide standard solution B and 0.5 mL of water. Seal the vial, and mix. Heat the mixture at 70 for 45 minutes to obtain Standard solution B.
for 45 minutes to obtain Standard solution B.
Test solution—
Transfer about 1 g of Diethylene Glycol Monoethyl Ether, accurately weighed, to a 10-mL pressure headspace vial, add 1 mL of water, seal the vial, and mix. Heat the mixture at 70 for 45 minutes to obtain the Test solution.
for 45 minutes to obtain the Test solution.
Chromatographic system
(see Chromatography  621
621 )—[note—The use of a headspace apparatus that automatically transfers a measured amount of headspace is allowed.] The gas chromatograph is equipped with a flame-ionization detector, maintained at about 250
)—[note—The use of a headspace apparatus that automatically transfers a measured amount of headspace is allowed.] The gas chromatograph is equipped with a flame-ionization detector, maintained at about 250 , and a 0.32-mm × 30-m glass or quartz capillary column bonded with a 1.0-µm layer of phase G1. The injection port temperature is maintained at about 150
, and a 0.32-mm × 30-m glass or quartz capillary column bonded with a 1.0-µm layer of phase G1. The injection port temperature is maintained at about 150 . The column temperature is maintained at 50
. The column temperature is maintained at 50 for 5 minutes after injection, then programmed to increase at the rate of 5
for 5 minutes after injection, then programmed to increase at the rate of 5 per minute to 180
per minute to 180 , then at the rate of 30
, then at the rate of 30 per minute to 230
per minute to 230 and to maintain this temperature for 5 minutes. The carrier gas is helium flowing at a rate of about 1 mL per minute. Chromatograph the gaseous phase of Standard solution A, and record the peak responses as directed for Procedure, adjusting the sensitivity of the system so that the peak heights of the two principal peaks in the chromatogram are not less than 15% of the full scale of the recorder: the relative retention times are about 0.94 for acetaldehyde and 1.0 for ethylene oxide; the resolution, R, between the acetaldehyde and ethylene oxide peaks is not less than 2.0; and the relative standard deviation for replicate injections is not more than 15%.
and to maintain this temperature for 5 minutes. The carrier gas is helium flowing at a rate of about 1 mL per minute. Chromatograph the gaseous phase of Standard solution A, and record the peak responses as directed for Procedure, adjusting the sensitivity of the system so that the peak heights of the two principal peaks in the chromatogram are not less than 15% of the full scale of the recorder: the relative retention times are about 0.94 for acetaldehyde and 1.0 for ethylene oxide; the resolution, R, between the acetaldehyde and ethylene oxide peaks is not less than 2.0; and the relative standard deviation for replicate injections is not more than 15%.
Procedure—
[note—Use peak areas where peak responses are indicated.] Using a heated, gas-tight gas chromatographic syringe, separately inject equal volumes (about 1 mL) of the gaseous headspace of Standard solution B and the Test solution into the chromatograph, record the chromatograms, and measure the peak responses: the mean area of the ethylene oxide peak in the chromatogram obtained from the Test solution is not greater than half the mean area of the corresponding peak in the chromatogram obtained from Standard solution B. Calculate the amount of ethylene oxide in the portion of Diethylene Glycol Monoethyl Ether taken by the formula:
rU / [(rSWU)  (rUWS)]
(rUWS)]
in which rU and rS are the ethylene oxide peak responses obtained from the Test solution and Standard solution B, respectively; and WU and WS are the weights, in g, of Diethylene Glycol Monoethyl Ether taken to prepare the Test solution and Standard solution B, respectively: not more than 1 µg per g is found.
Limit of 2-methoxyethanol, 2-ethoxyethanol, ethylene glycol, and diethylene glycol—
System suitability solution
and Chromatographic system—Proceed as directed in the Assay.
Procedure—
Proceed as directed in the Assay. Calculate the percentage of 2-methoxyethanol in the portion of Diethylene Glycol Monoethyl Ether taken by the formula:
100(ri0 / rs)
in which ri0 is the peak response for 2-methoxyethanol; and rs is the sum of the responses of all the peaks: not more than 50 µg per g of 2-methoxyethanol is found. Calculate the percentage of 2-ethoxyethanol in the portion of Diethylene Glycol Monoethyl Ether taken by the formula:
100(ri1 / rs)
in which ri1 is the peak response for 2-ethoxyethanol: not more than 160 µg per g of 2-ethoxyethanol is found. Calculate the percentage of ethylene glycol in the portion of Diethylene Glycol Monoethyl Ether taken by the formula:
100(ri2 / rs)
in which ri2 is the peak response for ethylene glycol: not more than 620 µg per g of ethylene glycol is found. Calculate the percentage of diethylene glycol in the portion of Diethylene Glycol Monoethyl Ether taken by the formula:
100(ri3 / rs)
in which ri3 is the peak response for diethylene glycol: not more than 150 µg per g of diethylene glycol is found.
Assay—
System suitability solution—
Prepare a solution in methanol containing about 1 mg each of 2-methoxyethanol, 2-ethoxyethanol, ethylene glycol, diethylene glycol, and USP Diethylene Glycol Monoethyl Ether RS per mL.
Chromatographic system
(see Chromatography  621
621 )—The gas chromatograph is equipped with a flame-ionization detector, maintained at a temperature of about 275
)—The gas chromatograph is equipped with a flame-ionization detector, maintained at a temperature of about 275 , and a 0.32-mm × 30-m fused-silica column bonded with a 1.0-µm layer of phase G46. The column temperature is programmed to be maintained at about 120
, and a 0.32-mm × 30-m fused-silica column bonded with a 1.0-µm layer of phase G46. The column temperature is programmed to be maintained at about 120 for 1 minute, then to increase to 225
for 1 minute, then to increase to 225 at a rate of 12
at a rate of 12 per minute, and to be maintained at 225
per minute, and to be maintained at 225 for 2 minutes. The injection port temperature is maintained at about 250
for 2 minutes. The injection port temperature is maintained at about 250 . Helium is used as the carrier gas at a flow rate of about 2.2 mL per minute. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.40 for 2-methoxyethanol, 0.43 for 2-ethoxyethanol, 0.50 for ethylene glycol, 0.93 for diethylene glycol monoethyl ether, and 1.0 for diethylene glycol; the resolution, R, between 2-ethoxyethanol and ethylene glycol is not less than 2.0; and the relative standard deviation for replicate injections is not more than 2.0% determined from diethylene glycol monoethyl ether.
. Helium is used as the carrier gas at a flow rate of about 2.2 mL per minute. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.40 for 2-methoxyethanol, 0.43 for 2-ethoxyethanol, 0.50 for ethylene glycol, 0.93 for diethylene glycol monoethyl ether, and 1.0 for diethylene glycol; the resolution, R, between 2-ethoxyethanol and ethylene glycol is not less than 2.0; and the relative standard deviation for replicate injections is not more than 2.0% determined from diethylene glycol monoethyl ether.
Procedure—
Inject a volume (about 0.5 µL) of Diethylene Glycol Monoethyl Ether into the chromatograph, record the chromatogram, and measure the areas for the major peaks. Calculate the percentage of C6H14O3 in the portion of Diethylene Glycol Monoethyl Ether taken by the formula:
100(A / B)
in which A is the diethylene glycol monoethyl ether peak response; and B is the sum of the responses of all the peaks in the chromatogram.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Robert H. Lafaver, B.A.
Scientist 1-301-816-8335 |
(EM105) Excipient Monographs 1 |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 1226
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.