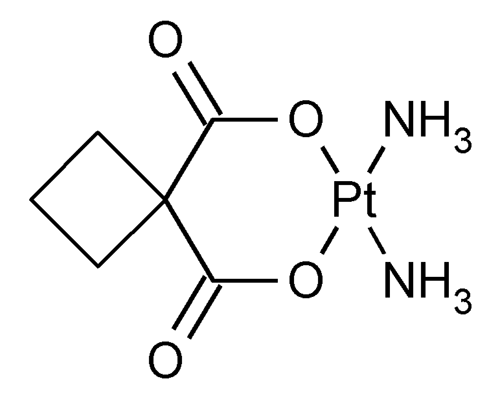
Carboplatin
Platinum, diammine[1,1-cyclobutanedicarboxylato(2-)-O,O¢]-, (SP-4-2).
cis-Diammine(1,1-cyclobutanedicarboxylato)platinum
» Carboplatin contains not less than 98.0 percent and not more than 102.0 percent of C6H12N2O4Pt, calculated on the anhydrous basis.
Caution: Great care should be taken in handling Carboplatin because it is a suspected carcinogen.
Packaging and storage—
Preserve in tight containers, protected from light.
Crystallinity  695
695 :
meets the requirements.
:
meets the requirements.
pH  791
791 :
between 5.0 and 7.0, in a solution in water containing 10 mg per mL.
:
between 5.0 and 7.0, in a solution in water containing 10 mg per mL.
Water, Method I  921
921 :
not more than 0.5%, anhydrous formamide being used as the solvent.
:
not more than 0.5%, anhydrous formamide being used as the solvent.
Transmittance—
Transfer about 100 mg of Carboplatin, accurately weighed, to a 10-mL volumetric flask, dissolve in 6 mL of water, dilute with water to volume, and mix. Determine the percent transmittance in 1-cm cells at a wavelength of 440 nm, using water as the blank: not less than 97% transmittance is observed.
Water-insoluble matter—
Transfer about 1 g of carboplatin, accurately weighed, to a 150-mL beaker. Add 100 mL of water, and dissolve by stirring with a stirring bar for 30 minutes. With aid of suction, filter through a tared filtering crucible. Rinse the beaker with water, and transfer the rinsings to the crucible. Dry the crucible at 130 ± 10 to constant weight: not more than 0.5% is found.
to constant weight: not more than 0.5% is found.
Limit of 1,1-cyclobutanedicarboxylic acid—
Reagent A—
Dissolve 8.5 g of tetrabutylammonium hydrogen sulfate in 80 mL of water. Add 3.4 mL of phosphoric acid, and adjust with 10 N sodium hydroxide to a pH of 7.55.
Mobile phase—
Add 20 mL of Reagent A to a mixture of 880 mL of water and 100 mL of acetonitrile, mix, filter, and degas. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard solution—
Dissolve an accurately weighed quantity of 1,1-cyclobutanedicarboxylic acid in Mobile phase to obtain a solution having a known concentration of about 0.5 mg per mL. Transfer 2.0 mL of this solution to a 200-mL volumetric flask, dilute with Mobile phase to volume, and mix.
System suitability solution—
Mix 1.0 mL of the Standard solution with 1.0 mL of Standard preparation, prepared as directed in the Assay.
Test solution—
Transfer about 50 mg of Carboplatin, accurately weighed, to a 50-mL volumetric flask. Dissolve in and dilute with Mobile phase to volume, and mix.
Chromatographic system
(see Chromatography  621
621 )—The liquid chromatograph is equipped with a 220-nm detector and a 4.0-mm × 30-cm column that contains packing L1. The flow rate is about 2 mL per minute. Chromatograph replicate injections (about 100 µL) of the System suitability solution, record the chromatograms, and measure the peak responses: the relative retention times are about 0.65 for carboplatin and 1.0 for 1,1-cyclobutanedicarboxylic acid; the column efficiency, determined from the 1,1-cyclobutanedicarboxylic acid peak, is not less than 1500 theoretical plates; the resolution, R, between carboplatin and 1,1-cyclobutanedicarboxylic acid peaks is not less than 2.5; and the relative standard deviation for replicate injections is not more than 10%.
)—The liquid chromatograph is equipped with a 220-nm detector and a 4.0-mm × 30-cm column that contains packing L1. The flow rate is about 2 mL per minute. Chromatograph replicate injections (about 100 µL) of the System suitability solution, record the chromatograms, and measure the peak responses: the relative retention times are about 0.65 for carboplatin and 1.0 for 1,1-cyclobutanedicarboxylic acid; the column efficiency, determined from the 1,1-cyclobutanedicarboxylic acid peak, is not less than 1500 theoretical plates; the resolution, R, between carboplatin and 1,1-cyclobutanedicarboxylic acid peaks is not less than 2.5; and the relative standard deviation for replicate injections is not more than 10%.
Procedure—
Separately inject equal volumes (about 100 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the peak responses for the 1,1-cyclobutanedicarboxylic acid peaks. Calculate the percentage of 1,1-cyclobutanedicarboxylic acid in the portion of Carboplatin taken by the formula:
5(C / W)(rU / rS)
in which C is the concentration, in µg per mL, of 1,1-cyclobutanedicarboxylic acid in the Standard solution, W is the weight, in mg, of Carboplatin taken to prepare the Test solution, and rU and rS are the peak responses for 1,1-cyclobutanedicarboxylic acid obtained from the Test solution and the Standard solution, respectively: not more than 0.5% is found.
Chromatographic purity—
Mobile phase, Chromatographic system, and Procedure—
Proceed as directed in the Assay.
Standard solution—
Quantitatively dilute a volume of the Standard preparation, prepared as directed in the Assay, with water to obtain a solution having a known concentration of about 2.5 µg of USP Carboplatin RS per mL.
Test solution—
Use the Assay preparation.
Procedure—
Separately inject equal volumes (about 10 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the peak responses. The sum of the peak responses, excluding the carboplatin and 1,1-cyclobutanedicarboxylic acid responses, from the Test solution, is not more than 2 times the carboplatin response from the Standard solution, and no single peak response is greater than that of the carboplatin peak from the Standard solution: not more than 0.25% of any individual impurity is found, and the sum of all impurities is not more than 0.5%.
Platinum content—
[note—Thoroughly cleanse all glassware with nitric acid and rinse with water to prevent “mirroring” of platinum precipitate.] Transfer about 0.25 g of Carboplatin, accurately weighed, to a 600-mL beaker. Add 400 mL of water, and slowly dissolve by heating almost to the boiling point, stirring frequently with a glass rod. Proceed as directed in the test for Platinum content under Cisplatin, beginning with “When solution is complete.” The weight of the platinum so obtained is between 52.0% and 53.0% of the carboplatin taken, calculated on the anhydrous basis.
Assay—
Mobile phase—
Prepare a filtered and degassed mixture of acetonitrile and water (87:13). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard preparation—
Dissolve an accurately weighed quantity of USP Carboplatin RS in water, and quantitatively dilute with water to obtain a solution having a known concentration of about 1 mg per mL. [note—Use this solution within 2 hours.]
Assay preparation—
Transfer about 50 mg of Carboplatin, accurately weighed, to a 50-mL volumetric flask, dissolve in and dilute with water to volume, and mix. [note—Use this solution within 2 hours.]
Chromatographic system
(see Chromatography  621
621 )—The liquid chromatograph is equipped with a 230-nm detector and a 4.0-mm × 30-cm column that contains packing L8. The flow rate is about 2.0 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the capacity factor, k¢, is not less than 3.0, the column efficiency is not less than 2500 theoretical plates, the tailing factor is not more than 2.5, and the relative standard deviation for replicate injections is not more than 1.2%.
)—The liquid chromatograph is equipped with a 230-nm detector and a 4.0-mm × 30-cm column that contains packing L8. The flow rate is about 2.0 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the capacity factor, k¢, is not less than 3.0, the column efficiency is not less than 2500 theoretical plates, the tailing factor is not more than 2.5, and the relative standard deviation for replicate injections is not more than 1.2%.
Procedure—
Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of C6H12N2O4Pt in the portion of Carboplatin taken by the formula:
50C(rU / rS)
in which C is the concentration, in mg per mL, of USP Carboplatin RS in the Standard preparation, and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
Chromatographic Column—
| Topic/Question | Contact | Expert Committee |
| Monograph | Feiwen Mao, M.S.
Scientist 1-301-816-8345 |
(MDOOD05) Monograph Development-Ophthalmics Oncologics and Dermatologicals |
| Reference Standards | Lili Wang, Technical Services Scientist 1-301-816-8129 RSTech@usp.org |
USP32–NF27 Page 1799
Chromatographic columns text is not derived from, and not part of, USP 32 or NF 27.