- British Pharmacopoeia Volume IV
- Appendices
Appendix V G. Determination of Weight per Millilitre, Density, Relative Density and Apparent Density |
The weight per millilitre of a liquid is the weight in g of 1 ml of a liquid when weighed in air at 20°, unless otherwise specified in the monograph.
The weight per millilitre is determined by dividing the weight in air, expressed in g, of the quantity of liquid that fills a pycnometer at the specified temperature by the capacity, expressed in ml, of the pycnometer at the same temperature. The capacity of the pycnometer is ascertained from the weight in air, expressed in g, of the quantity of water required to fill the pycnometer at that temperature. The weight of a litre of water at specified temperatures when weighed against brass weights in air of density 0.0012 g per ml is given in the following table. Ordinary deviations in the density of air from the above value, here taken as the mean, do not affect the result of a determination in the significant figures prescribed for Pharmacopoeial substances.

The density, ρ20, of a substance is the ratio of its mass to its volume at 20°. It is expressed in kg m–3.
The density is determined by dividing the weight in air of the quantity of the liquid being examined that fills a pycnometer at 20° by the weight in air of water required to fill the pycnometer after making allowance for the thrust of the air.
The density is calculated from the expression
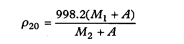
where |
M 1 |
= |
weight in air (apparent mass) in grams of the substance being examined, |
M 2 |
= |
weight in air (apparent mass) in grams of water, |
|
A |
= |
the correction factor for the thrust of the air, 0.0012M 2 |
|
998.2 |
= |
the density of water at 20° in kg m–3. |
In most cases, the correction for the thrust of the air may be disregarded.
The relative density 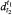 of a substance is the ratio of the mass of a certain volume of a substance at temperature t1 to the mass of an equal volume of water at temperature t2.
of a substance is the ratio of the mass of a certain volume of a substance at temperature t1 to the mass of an equal volume of water at temperature t2.
Unless otherwise indicated, the relative density 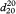 is used. Relative density is also commonly expressed as
is used. Relative density is also commonly expressed as 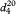 . Density ρ20, defined as the mass of a unit volume of the substance at 20 °C may also be used, expressed in kilograms per cubic metre or grams per cubic centimetre (1 kg·m-3 = 10-3 g·cm-3). These quantities are related by the following equations where density is expressed in grams per cubic centimetre:
. Density ρ20, defined as the mass of a unit volume of the substance at 20 °C may also be used, expressed in kilograms per cubic metre or grams per cubic centimetre (1 kg·m-3 = 10-3 g·cm-3). These quantities are related by the following equations where density is expressed in grams per cubic centimetre:
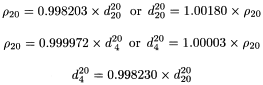
Relative density or density are measured with the precision to the number of decimals prescribed in the monograph using a density bottle (solids or liquids), a hydrostatic balance (solids), a hydrometer (liquids) or a digital density meter with an oscillating transducer (liquids and gases). When the determination is made by weighing, the buoyancy of air is disregarded, which may introduce an error of 1 unit in the 3rd decimal place. When using a density meter, the buoyancy of air has no influence.
Oscillating transducer density meter The apparatus consists of:
- — a U-shaped tube, usually of borosilicate glass, which contains the liquid to be examined;
- — a magneto-electrical or piezo-electrical excitation system that causes the tube to oscillate as a cantilever oscillator at a characteristic frequency depending on the density of the liquid to be examined;
- — a means of measuring the oscillation period (T), which may be converted by the apparatus to give a direct reading of density, or used to calculate density using the constants A and B described below.
The resonant frequency (f) is a function of the spring constant (c) and the mass (m) of the system:
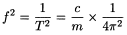
Hence:
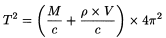
M |
= |
mass of the tube, |
V |
= |
inner volume of the tube. |
Introduction of 2 constants A = c /(4π2 × V) and B = M/V, leads to the classical equation for the oscillating transducer:
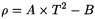
The constants A and B are determined by operating the instrument with the U-tube filled with 2 different samples of known density, for example, degassed water R and air. Control measurements are made daily using degassed water R. The results displayed for the control measurement using degassed water R shall not deviate from the reference value (ρ20 = 0.998203 g·cm-3, 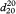 = 1.000000) by more than its specified error. For example, an instrument specified to ± 0.0001 g·cm-3 shall display 0.9982 ± 0.0001 g·cm-3 in order to be suitable for further measurement. Otherwise a re-adjustment is necessary. Calibration with certified reference materials is carried out regularly. Measurements are made using the same procedure as for calibration. The liquid to be examined is equilibrated in a thermostat at 20 °C before introduction into the tube, if necessary, to avoid the formation of bubbles and to reduce the time required for measurement.
= 1.000000) by more than its specified error. For example, an instrument specified to ± 0.0001 g·cm-3 shall display 0.9982 ± 0.0001 g·cm-3 in order to be suitable for further measurement. Otherwise a re-adjustment is necessary. Calibration with certified reference materials is carried out regularly. Measurements are made using the same procedure as for calibration. The liquid to be examined is equilibrated in a thermostat at 20 °C before introduction into the tube, if necessary, to avoid the formation of bubbles and to reduce the time required for measurement.
Factors affecting accuracy include:
- — temperature uniformity throughout the tube,
- — non-linearity over a range of density,
- — parasitic resonant effects,
- — viscosity, whereby solutions with a higher viscosity than the calibrant have a density that is apparently higher than the true value.
The effects of non-linearity and viscosity may be avoided by using calibrants that have density and viscosity close to those of the liquid to be examined (± 5 per cent for density, ± 50 per cent for viscosity). The density meter may have functions for automatic viscosity correction and for correction of errors arising from temperature changes and non-linearity.
Precision is a function of the repeatability and stability of the oscillator frequency, which is dependent on the stability of the volume, mass and spring constant of the cell.
Density meters are able to achieve measurements with an error of the order of 1 × 10-3 g·cm-3 to 1 × 10-5 g·cm-3 and a repeatability of 1 × 10-4 g·cm-3 to 1 × 10-6 g·cm-3.
The term 'Apparent density' is used in the monographs for Dilute Ethanols, Industrial Methylated Spirit and Industrial Methylated Spirit (Ketone-free). It is defined as weight in air per unit volume and expressed in kg m–3. It is named 'density' in the Laboratory Alcohol Table for Laboratory Use (HM Customs and Excise 1979).
The apparent density is calculated from the following expression:
apparent density = 997.2 × 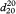
where 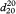 is the relative density of the substance being examined and 997.2 is the weight in air in kg of 1 cubic metre of water.
is the relative density of the substance being examined and 997.2 is the weight in air in kg of 1 cubic metre of water.