Bergmann Azlactone Peptide Synthesis
M. Bergmann et al., Ann. 449, 277 (1926).
Conversion of an acetylated amino acid and an aldehyde into an azlactone with an alkylene side chain, reaction with a second amino acid with ring opening and formation of an acylated unsaturated dipeptide, followed by catalytic hydrogenation and hydrolysis to the dipeptide:
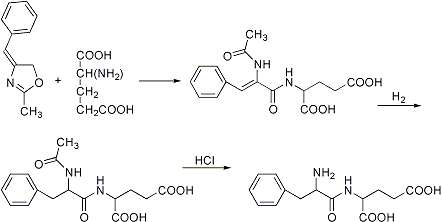
J. S. Fruton, Advan. Protein Chem. V, 15 (1949); S. Archer in Amino Acids and Proteins, D. M. Greenberg, Ed. (Thomas, Springfield, IL, 1951) p 181; H. D. Springall, The Structural Chemistry of Proteins (New York, 1954) p 29; E. Baltazzi, Quart. Rev. (London) 10, 235 (1956). Cf. Erlenmeyer-Plöchl Azlactone and Amino Acid Synthesis.