Erlenmeyer-Plöchl Azlactone and Amino Acid Synthesis
E. Erlenmeyer, Ann. 275, 1 (1893); J. Plöchl, Ber. 17, 1616 (1884).
Formation of azlactones by intramolecular condensation of acylglycines in the presence of acetic anhydride. The reaction of azlactones with carbonyl compounds followed by hydrolysis to the unsaturated α-acylamino acid and by reduction yields the amino acid; drastic hydrolysis gives the α-oxo acid:
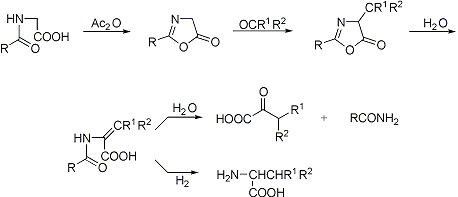
C. L. A. Schmidt, The Chemistry of the Amino Acids and Proteins (Springfield, IL, 1944) p 54; H. E. Carter, Org. React. 3, 198 (1946); M. Crawford, W. T. Little, J. Chem. Soc. 1959, 729; W. Steglich, Fortschr. Chem. Forsch. 12, 84 (1969); J. Cornforth, D. Ming-hui, J. Chem. Soc. Perkin Trans. I 1991, 2183; A. P. Combs, R. W. Armstrong, Tetrahedron Letters 33, 6419 (1992). Cf. Bergmann Azlactone Peptide Synthesis; Perkin Reaction.