| 参考文献No. | 45290 |
| 标题: | Macrocyclic peptides active against the hepatitis C virus |
| 作者: | Halmos, T.; Llinas-Brunet, M.; Faucher, A.-M.; Ghiro, E.; Goudreau, N.; Tsantrizos, Y.S.; Cameron, D.R. (Boehringer Ingelheim (Canada) Ltd.) |
| 来源: | EP 1169339; JP 2002542160; WO 0059929 |
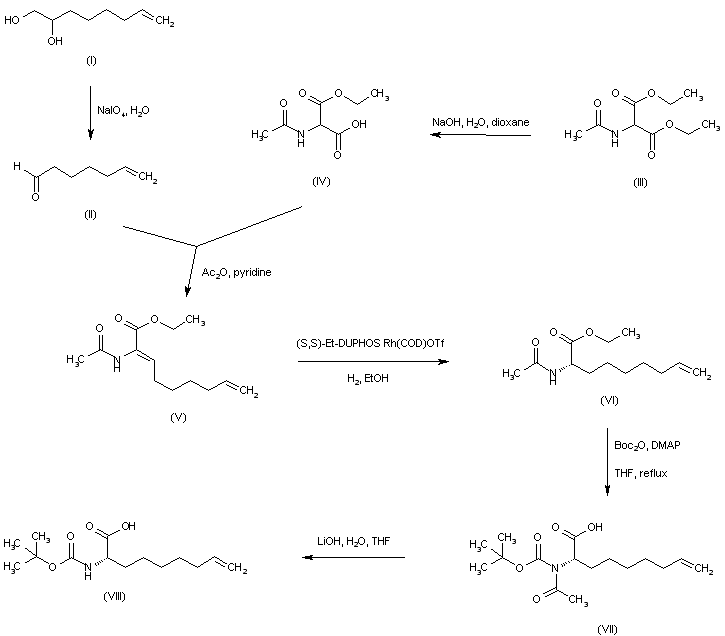 |
| 合成路线图解说明: The aminoacid building block (VIII) can be prepared by two different ways. The oxidative cleavage of 7-octene-1,2-diol (I) by means of NaIO4 yields aldehyde (II). Partial hydrolysis of diethyl 2-acetamidomalonate (III) provides monoacid (IV), which is subjected to Knoevenagel-type condensation with 6-heptenal (II) in the presence of Ac2O and pyridine to afford enamide (V). Enantioselective hydrogenation of the enamide double bond to the (S)-amidoester (VI) is then achieved by using the Burk's method. In order to replace the N-acetyl protecting group of (VI), the acetamide nitrogen is protected with Boc2O, and the resulting imide (VII) is further hydrolyzed to the target N-Boc aminoacid (VIII) under standard basic conditions. |
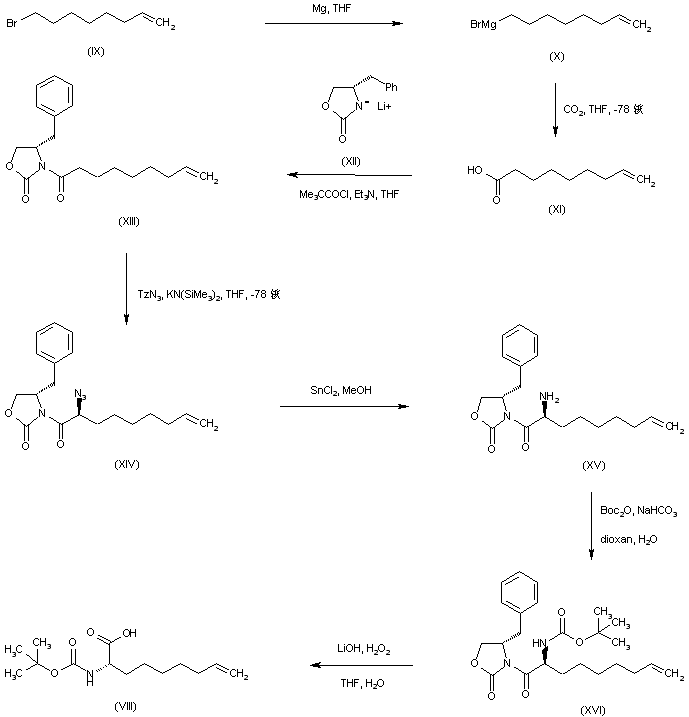 |
| 合成路线图解说明: Alternatively, 8-bromo-1-octene (IX) is converted into the corresponding Grignard reagent (X) which is further carboxylated by solid carbon dioxide in cold THF to produce acid (XI). Coupling of acid (XI) with the lithium derivative of (S)-4-benzyl-2-oxazolidinone (XII), via activation with pivalic anhydride, leads to the chiral N-acyl oxazolidinone (XIII). Stereoselective alpha-azidation of the potassium enolate of (XIII) with trizylazide forms the (S)-azide (XIV). Subsequent reduction of azide (XIV) employing SnCl2 leads to amine (XV), which is further protected as the N-Boc derivative (XVI) with Boc2O in the presence of NaHCO3. Then, hydrolysis of the chiral auxiliary of (XVI) by means of lithium hydroperoxide gives rise to the desired N-Boc aminoacid (VIII). |
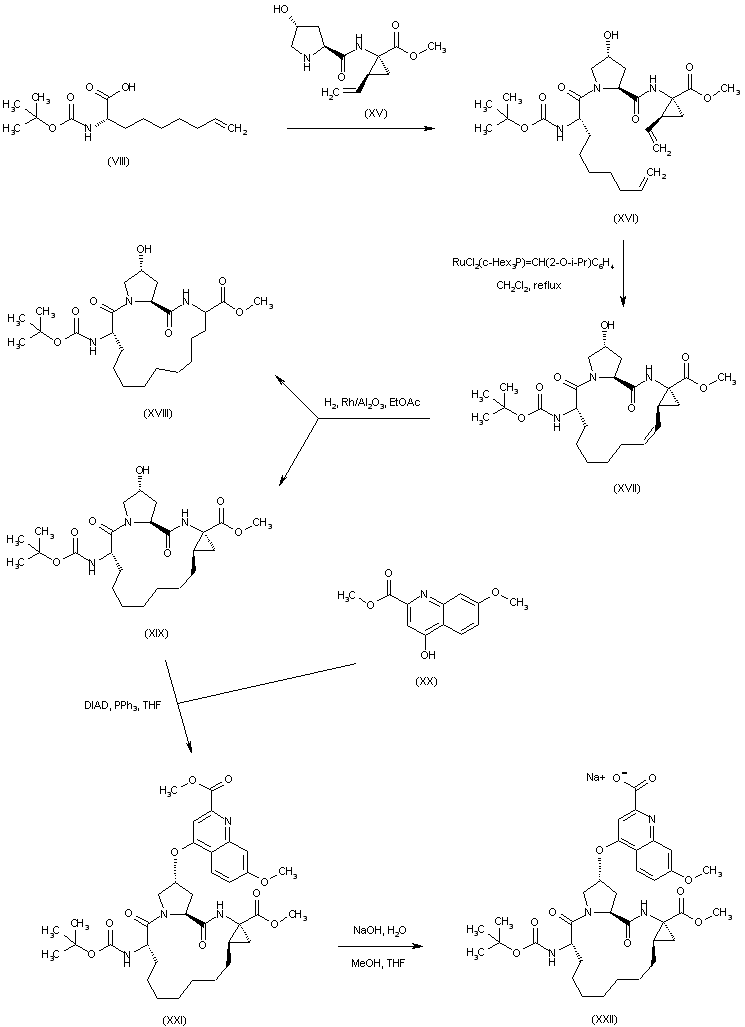 |
| 合成路线图解说明: Coupling between the Boc-aminoacid (VIII) and dipeptide ester (XV) affords the linear tripeptide (XVI). Macrocyclization of (XVI) to produce (XVII) is accomplished by intramolecular olefin metathesis in the presence of Hoveyda's catalyst. Subsequent catalytic hydrogenation of (XVII) leads to a mixture of the desired saturated macrocycle (XIX) and compound (XVIII), produced by hydrogenolysis of the cyclopropane ring. Mitsunobu coupling of (XIX) with methyl 4-hydroxy-7-methoxyquinoline-2-carboxylate (XX) gives rise to quinolinyl ether (XXI). The quinoline carboxylate group of (XXI) is then selectively hydrolyzed by NaOH to furnish the sodium carboxylate salt (XXII). |
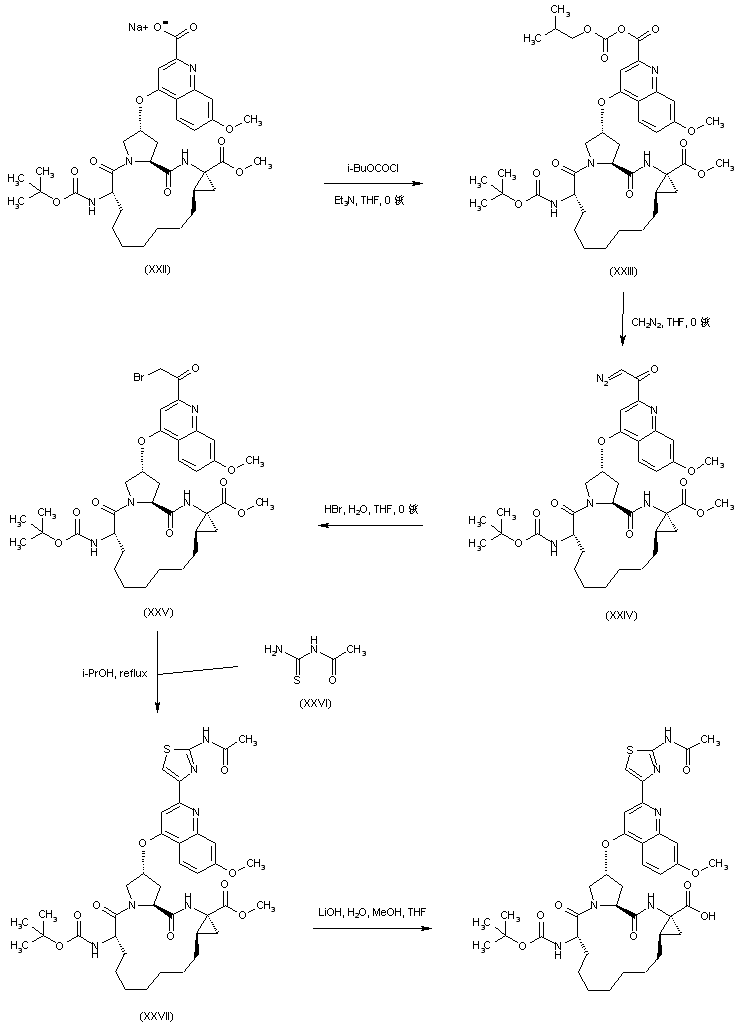 |
| 合成路线图解说明: The sodium carboxylate (XXII) is activated as the mixed anhydride (XXIII) by treatment with isobutyl chloroformate and triethylamine. Subsequent addition of diazomethane to the mixed anhydride (XXIII) leads to diazo ketone (XXIV), which is further transformed into bromo ketone (XXV) upon treatment with HBr. Cyclization of bromo ketone (XXV) with N-acetyl thiourea (XXVI) produces the thiazole derivative (XXVII). The methyl ester group of (XXVII) is finally hydrolyzed by means of LiOH to the target carboxylic acid. |