| 参考文献No. | 669927 |
| 标题: | Anticancer drug delivery systems: multi-loaded N-4-acyl poly(ethylene glycol) prodrugs of ara-C. II. Efficacy in ascites and solid tumors |
| 作者: | Choe, Y.H.; Conover, C.D.; Wu, D.; Royzen, M.; Gervacio, Y.; Borowski, V.; Mehlig, M.; Greenwald, R.B. |
| 来源: | J Control Release 2002,79(1-3),55 |
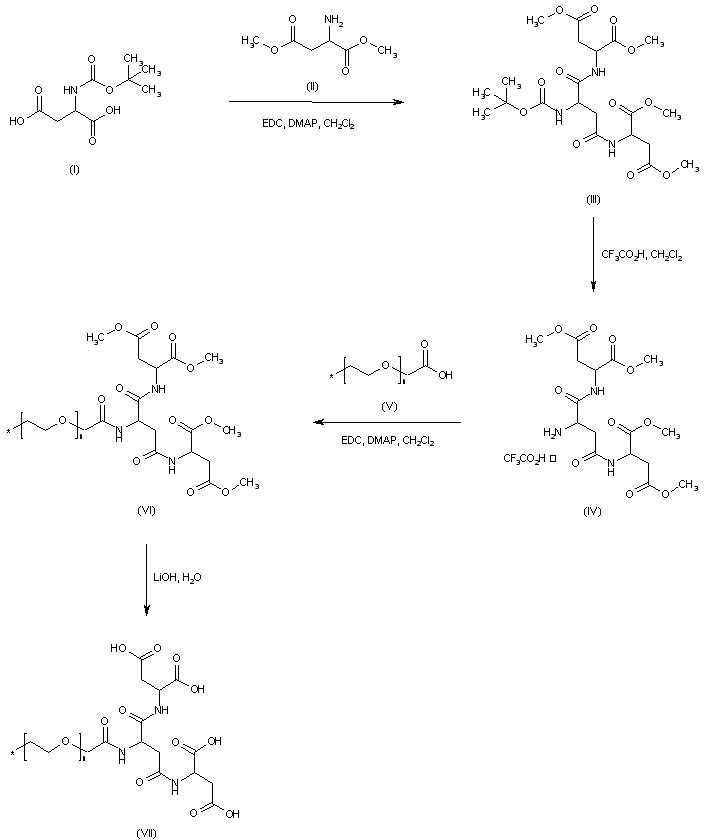 |
| 合成路线图解说明: Coupling between N-Boc-aspartic acid (I) and dimethyl aspartate (II) using EDC and DMAP affords tripeptide (III). Subsequent Boc group deprotection in (III) employing trifluoroacetic acid leads to amine (IV).This is then coupled to the polyethyleneglycol-acid (V) producing amide (VI). Basic hydrolysis of the ester groups of (VI) then gives the tetra-carboxylic acid scaffold (VII). |
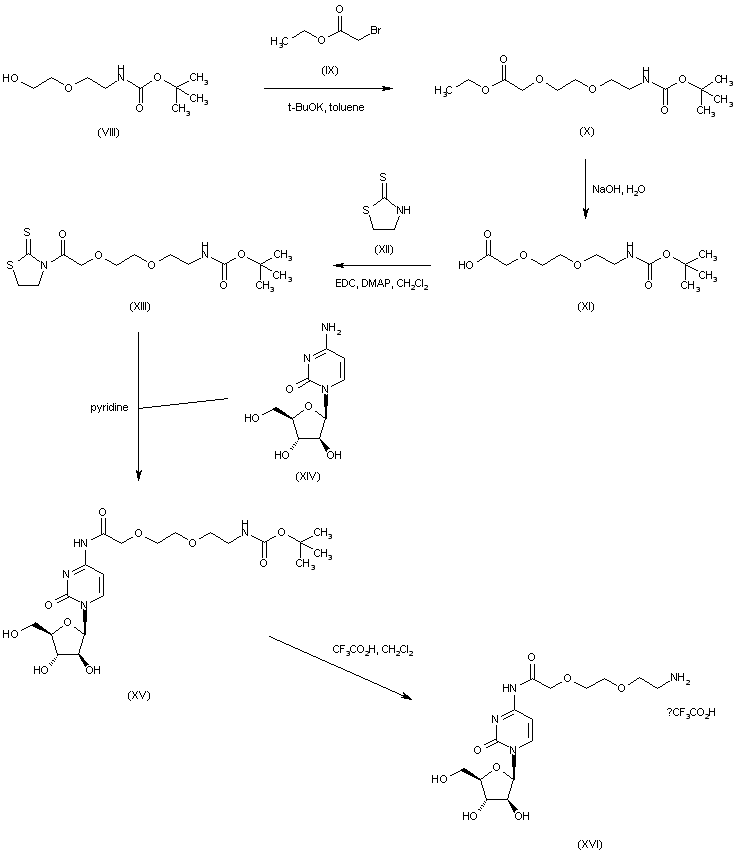 |
| 合成路线图解说明: The Boc-protected amino alcohol (VIII) is condensed with ethyl bromoacetate (IX) in the presence of potassium tert-butoxide to afford ether (X). After alkaline hydrolysis of the ethyl ester group of (X), the resultant carboxylic acid (XI) is activated as the thioimide (XIII) upon treatment with thiazolidine-2-thione (XII) and EDC. Coupling of the acyl thiazolidinone (XIII) with cytosine arabinoside (XIV) leads to the N-acyl cytosine derivative (XV). The Boc protecting group of (XV) is then removed under acidic conditions to furnish amine (XVI). |
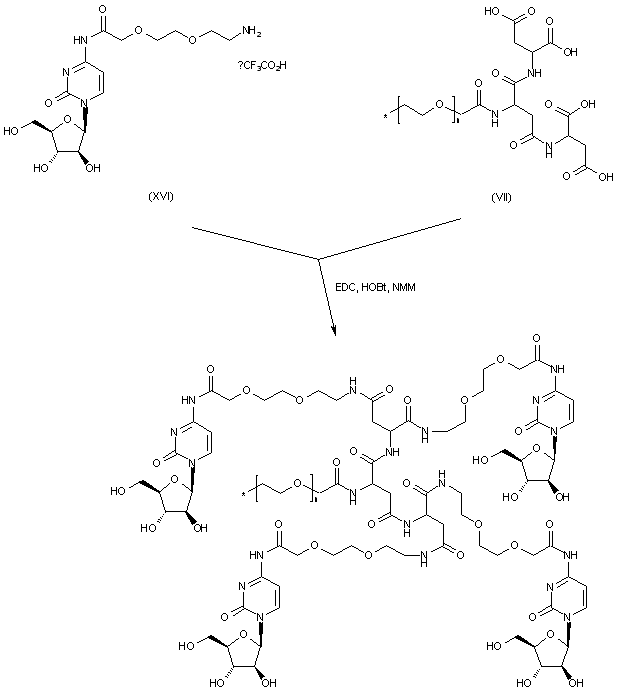 |
| 合成路线图解说明: Finally, condensation of the tetra-acid scaffold (VII) with the aminoacyl cytosine prodrug (XVI) gives rise to the title tetra-amide adduct. |