| 参考文献No. | 60925 |
| 标题: | Blood pool agents for nuclear magnetic resonance diagnostics |
| 作者: | Uggeri, F.; Brocchetta, M.; Visigalli, M.; Lux, G.; De Ha雗, C.; Serleti, M.; Palano, D.; Anelli, P.L.; Lattuada, L.; Morosini, P.; Gazzotti, O.; Manfredi, G. (Bracco SpA) |
| 来源: | CA 2355888; EP 1140209; US 6461588; WO 0038738 |
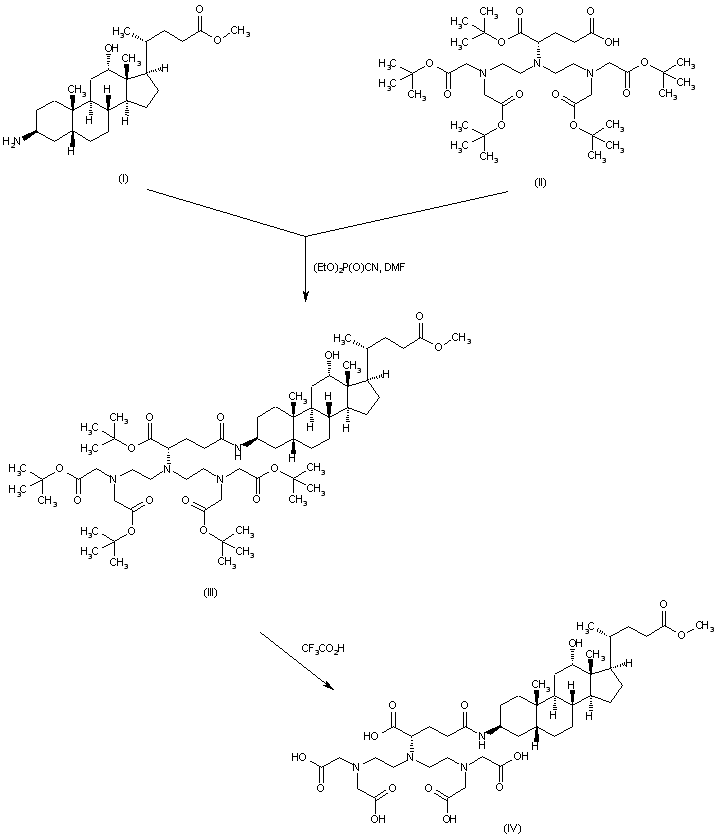 |
| 合成路线图解说明: 3-Amino-12-hydroxycholan-24-oic acid methyl ester (I) is coupled to the glutamic acid derivative (II) employing diethyl cyanophosphate to furnish amide (III). The tert-butyl ester groups are then cleaved by means of trifluoroacetic acid to provide the pentacarboxylic acid (IV) |
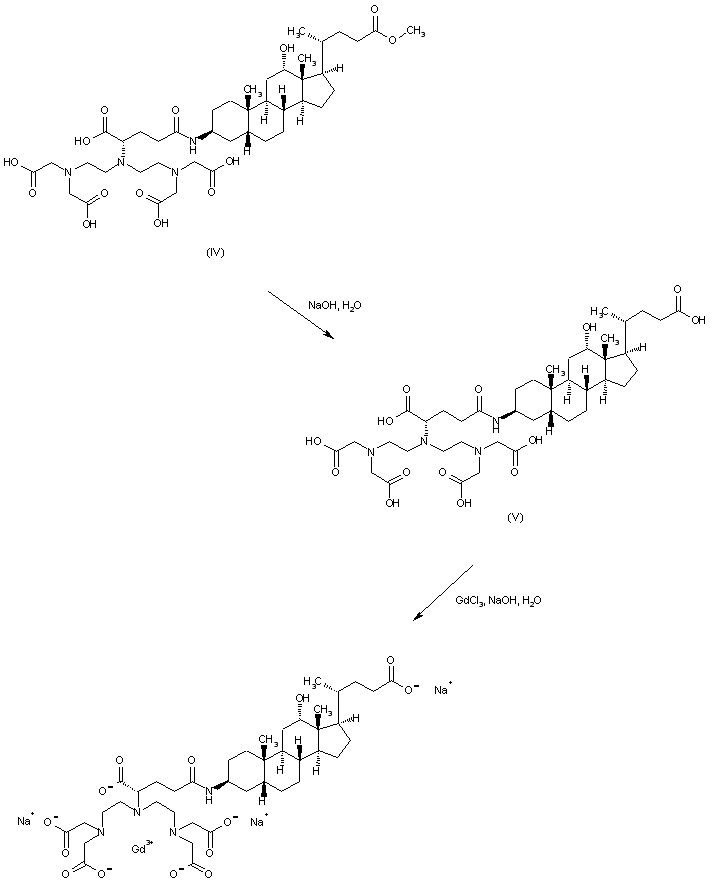 |
| 合成路线图解说明: The remaining methyl ester group of (IV) is saponified with aqueous NaOH to give acid (V). This compound is finally complexed with GdCl3 in alkaline solution to generate the title gadolinium chelate |
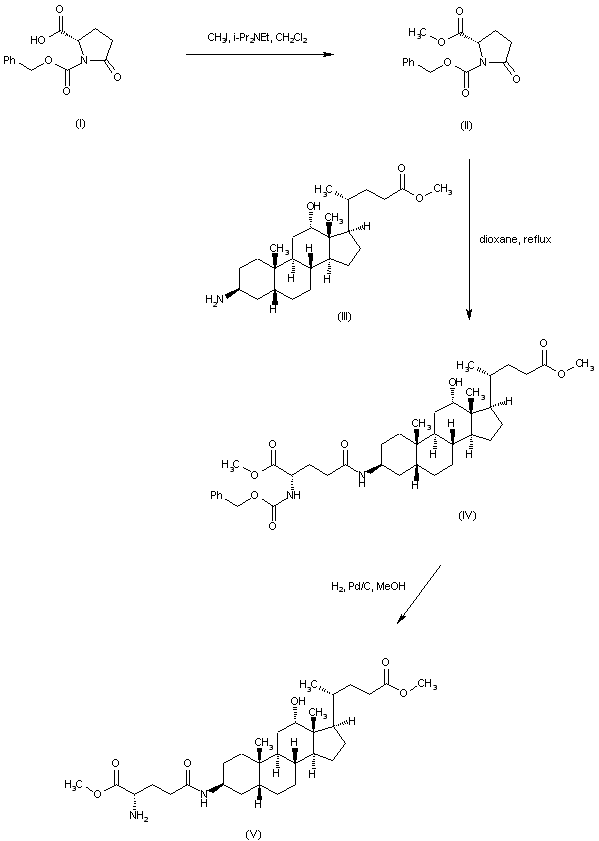 |
| 合成路线图解说明: In an alternative synthetic method for the precursor hexacarboxylic acid (VIII), N-benzyloxycarbonyl-L-pyroglutamic acid (I) is treated with iodomethane and diisopropylethylamine to produce the corresponding methyl ester (II). Condensation of (II) with 3-amino-12-hydroxycholan-24-oic acid methyl ester (III) leads to amide (IV). The N-Cbz group is then removed by hydrogenolysis over Pd/C to furnish (V) |
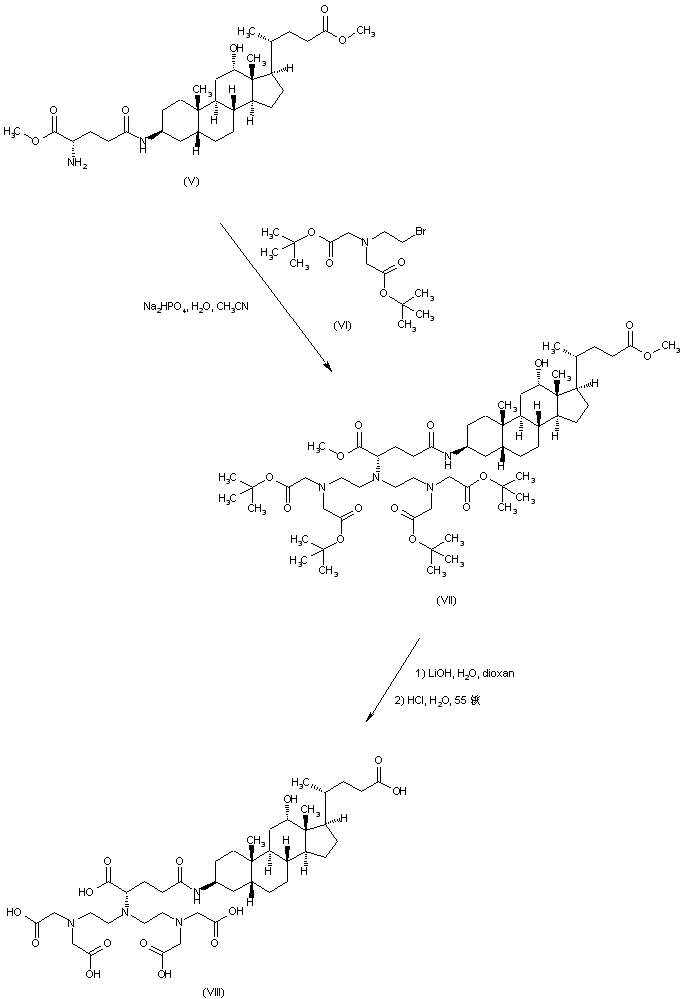 |
| 合成路线图解说明: Alkylation of amine (V) with N-(2-bromoethyl)aminodiacetic acid di-tert-butyl ester (VI) leads to (VII). Then, alkaline hydrolysis of the methyl ester groups of (VII), followed by acidic cleavage of the tert-butyl esters provides the precursor (VIII) |