| 参考文献No. | 48902 |
| 标题: | Inhibitors of neuraminidases |
| 作者: | Sham, H.L.; Kempf, D.J.; Maring, C.J.; Klein, L.L.; Zhao, C.; Wiedeman, P.E.; Hanessian, S.; Chen, Y.; Stewart, K.D.; Yeung, M.C.; Degoey, D.A.; Grampovnik, D.J.; Wang, S.; Gu, Y.G.; Xu, Y.; (Abbott Laboratories Inc.) |
| 来源: | WO 0128996 |
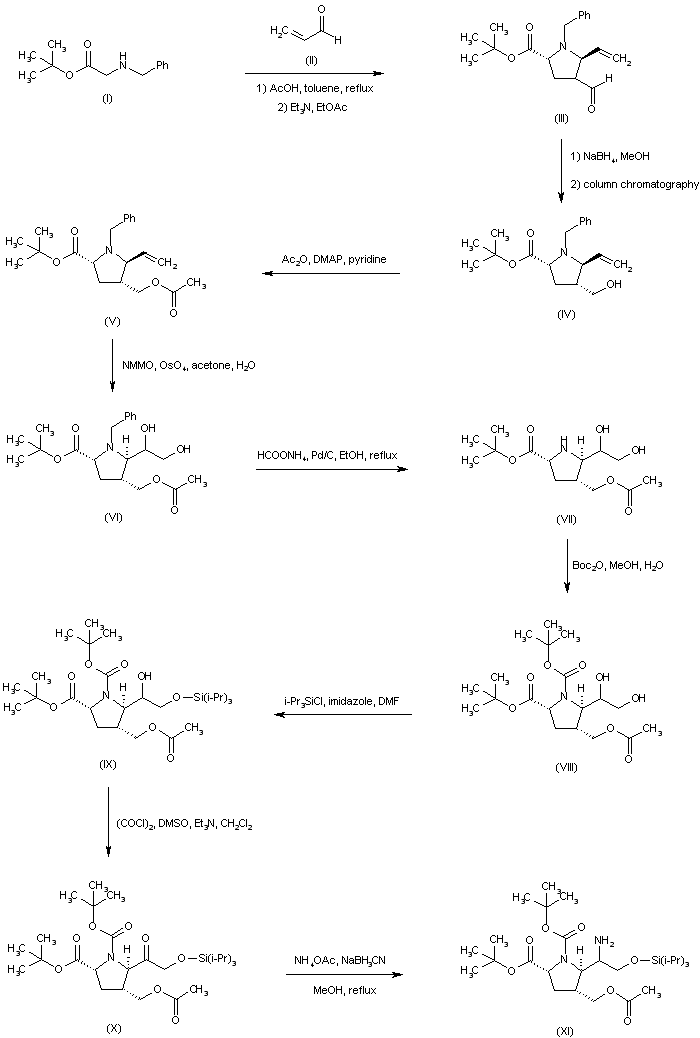 |
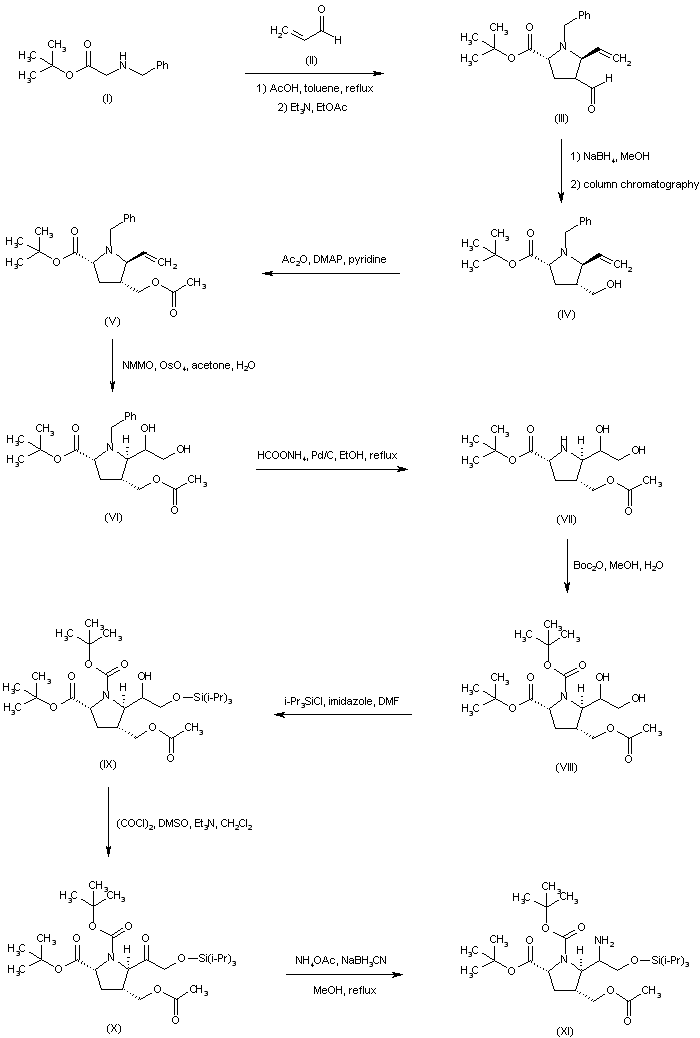
| 合成路线图解说明: The title compound was prepared by several synthetic routes: The condensation between N-benzylglycine t-butyl ester (I) and acrolein (II) furnished pyrrolidine (III) as a mixture of epimers at C-4. The mixture of aldehydes was further equilibrated to an 8:1 ratio by stirring the crude product with Et3N in EtOAc. Aldehyde reduction employing NaBH4 produced the corresponding mixture of alcohols, from which the desired isomer (IV) was isolated by column chromatography. After protection of the alcohol function as the acetate ester (V), dihydroxylation of the vinyl group of (V) with N-methylmorpholine N-oxide in the presence of OsO4 provided diol (VI). The N-benzyl group of (VI) was then removed by transfer hydrogenolysis with ammonium formate and Pd/C to give the secondary amine (VII), which was further converted to the N-Boc derivative (VIII). Selective silylation of the primary hydroxyl group of (VIII) employing triisopropylsilyl chloride and imidazole afforded the silyl ether (IX). The secondary hydroxyl group was then oxidized under Swern conditions to ketone (X). Reductive amination of (X) with ammonium acetate in the presence of NaBH3CN led to a mixture of epimeric amines (XI). |
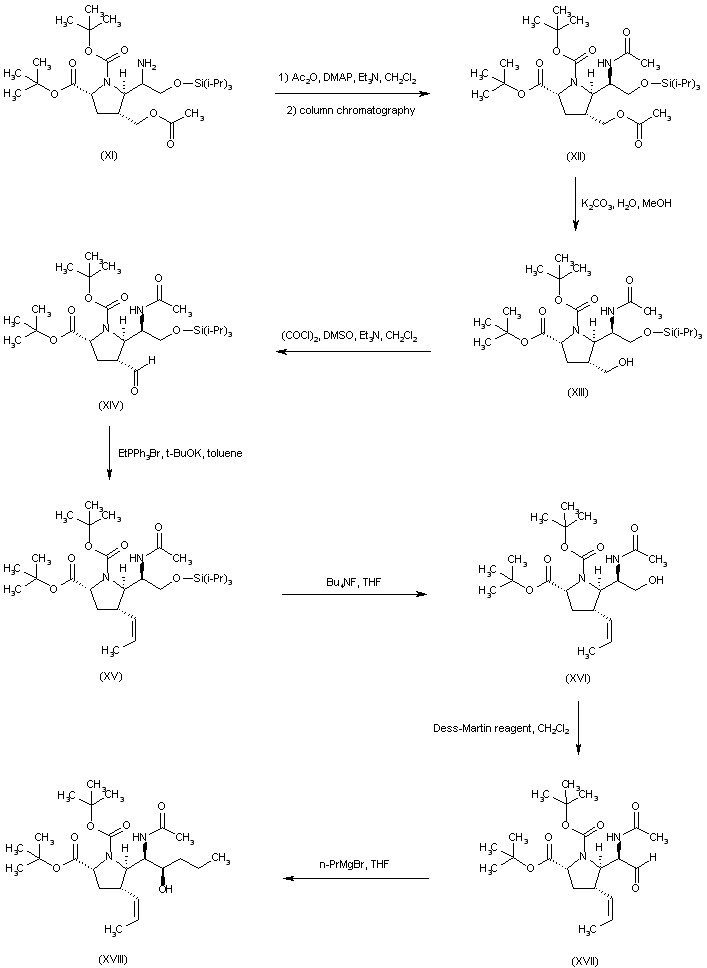 |
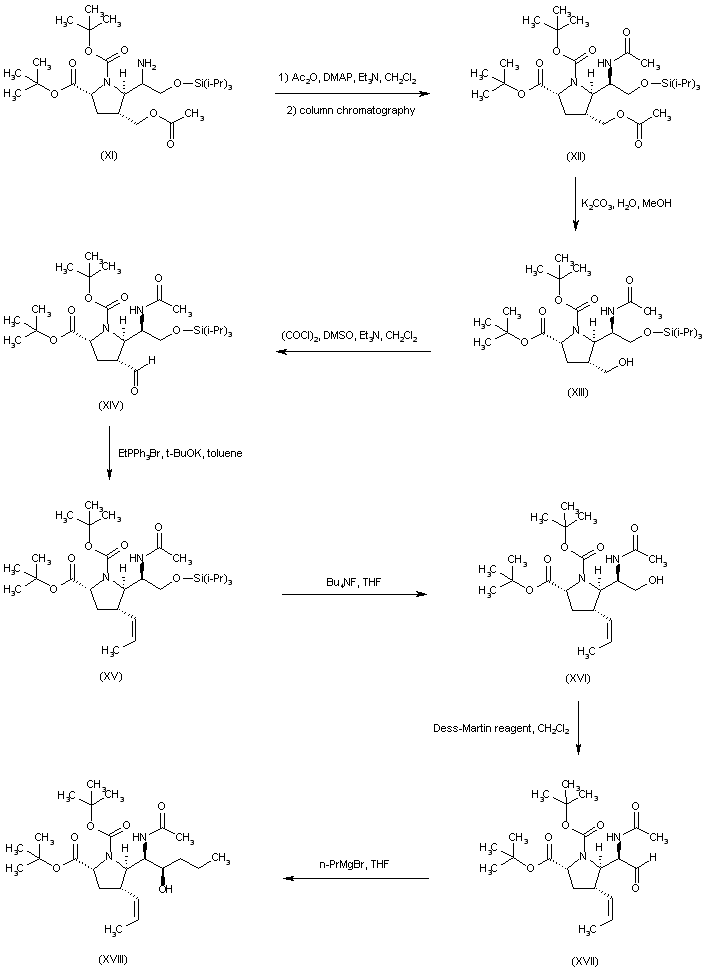
| 合成路线图解说明: The mixture of amines (XI) was converted to the respective acetamides, and the desired isomer (XII) was then isolated by column chromatography. Hydrolysis of the acetate ester function of (XII) provided alcohol (XIII), which was oxidized to aldehyde (XIV) under Swern conditions. Wittig reaction of aldehyde (XIV) with ethyl triphenylphosphonium bromide and potassium tert-butoxide led to the (Z)-olefin (XV). After removal of the O-silyl protecting group of (XV) by means of tetrabutylammonium fluoride, the resultant alcohol (XVI) was oxidized to aldehyde (XVII) employing the Dess-Martin periodinane reagent. Addition of propylmagnesium bromide to aldehyde (XVII) furnished the alcohol adduct (XVIII). |
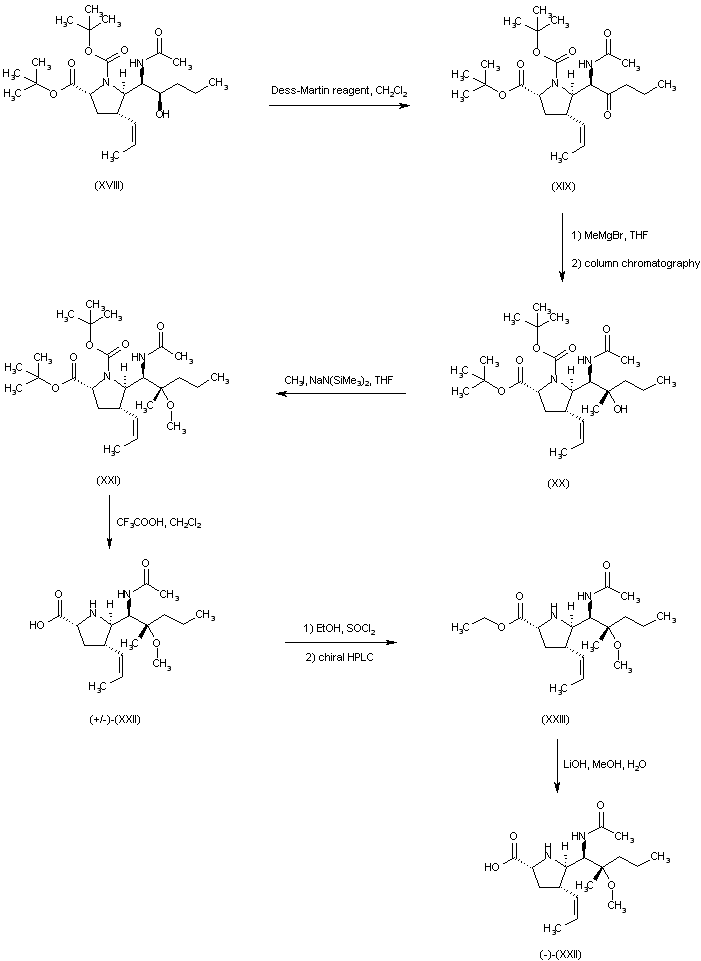 |
| 合成路线图解说明: Oxidation of alcohol (XVIII) employing the Dess-Martin periodinane reagent furnished ketone (XIX). Subsequent addition of methylmagnesium bromide to ketone (XIX) gave rise to a mixture of diastereoisomeric carbinols from which alcohol (XX) was isolated as the major isomer. The methyl ether (XXI) was prepared by alkylation of the sodium alkoxide of (XX) with iodomethane in THF. Trifluoroacetic acid-promoted cleavage of the tert-butyl ester and N-Boc protecting groups provided the title compound in the racemic form (+/-)-(XXII). The ethyl ester prepared by esterification of (+/-)-(XXII) with EtOH and SOCl2 was then resolved using chiral HPLC. The desired enantiomer (XXIII) was finally hydrolyzed with LiOH to the title levo carboxylic acid. |
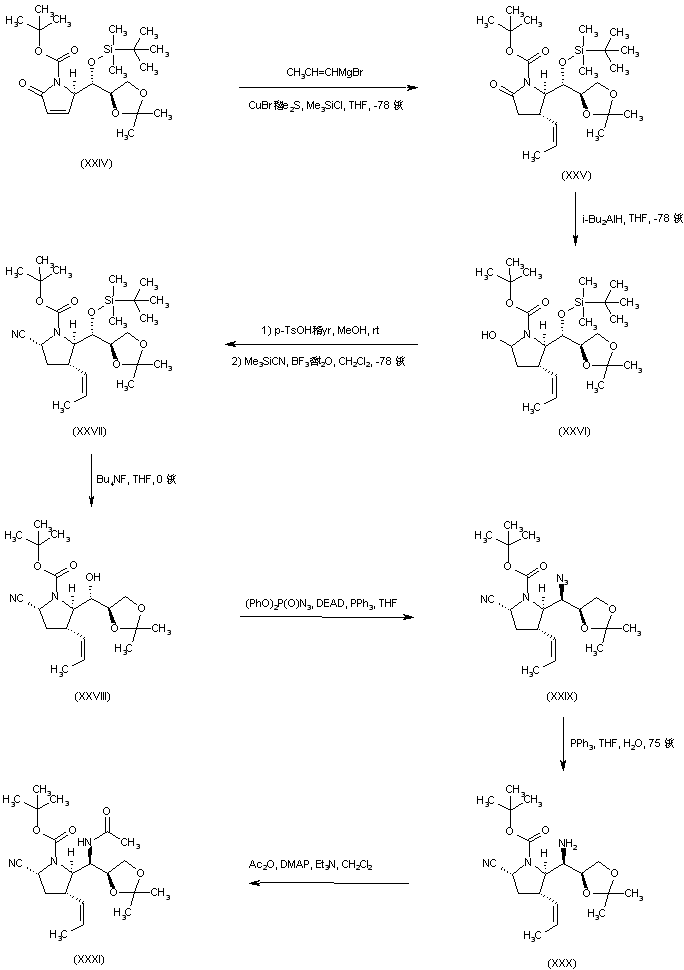 |
| 合成路线图解说明: In an alternative synthesis, copper-catalyzed conjugate addition of propenylmagnesium bromide to the chiral pyrrolidone (XXIV) afforded the propenyl pyrrolidinone (XXV). Reduction of the lactam function of (XXV) by means of DIBAL gave the 2-ydroxypyrrolidine (XXVI), which was reacted with trimethylsilyl cyanide and boron trifluoride to provide nitrile (XXVII). Alcohol (XXVIII), obtained by desilylation of (XXVII) with tetrabutylammonium fluoride, was converted to azide (XXIX) upon treatment with DPPA under Mitsunobu conditions. Azide (XXIX) was then reduced to the primary amine (XXX), employing triphenylphosphine in moist THF. Subsequent acylation with Ac2O furnished acetamide (XXXI). |
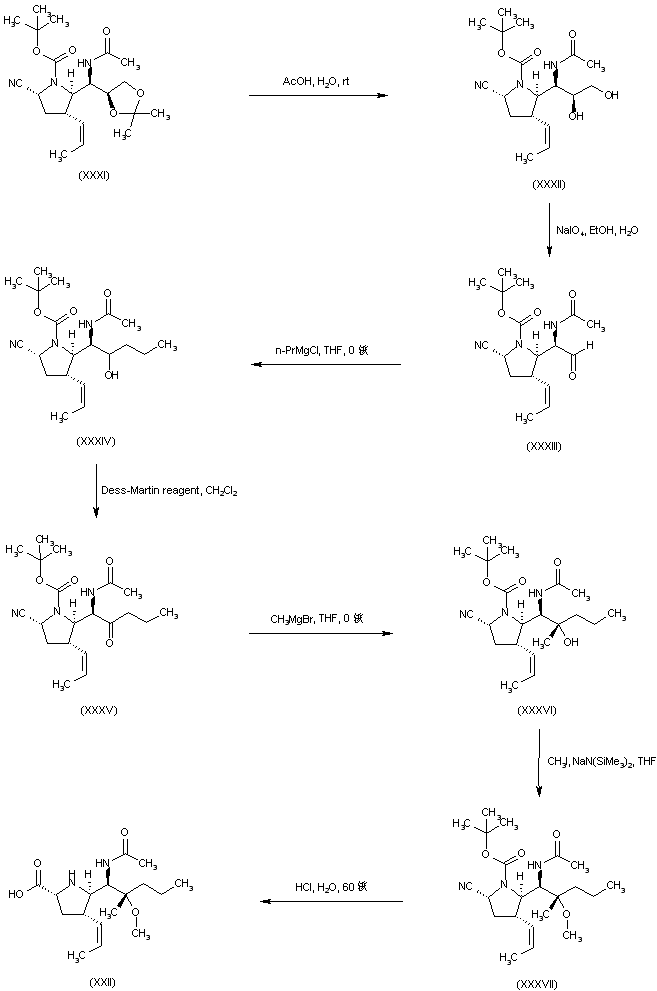 |
| 合成路线图解说明: Acidic hydrolysis of the acetonide (XXXI) gave rise to diol (XXXII), which was subjected to oxidative cleavage in the presence of NaIO4, yielding aldehyde (XXXIII). Addition of propylmagnesium chloride to aldehyde (XXXIII) furnished alcohol (XXXIV). After oxidation of (XXXIV) to the corresponding ketone (XXXV), addition of methylmagnesium bromide provided carbinol (XXXVI). The methyl ether (XXXVII) was prepared by alkylation of the sodium alkoxide of (XXXVI) with iodomethane. Finally, hydrolysis of the cyano group of (XXXVII) with concomitant Boc group cleavage under acidic conditions led to the title compound. |
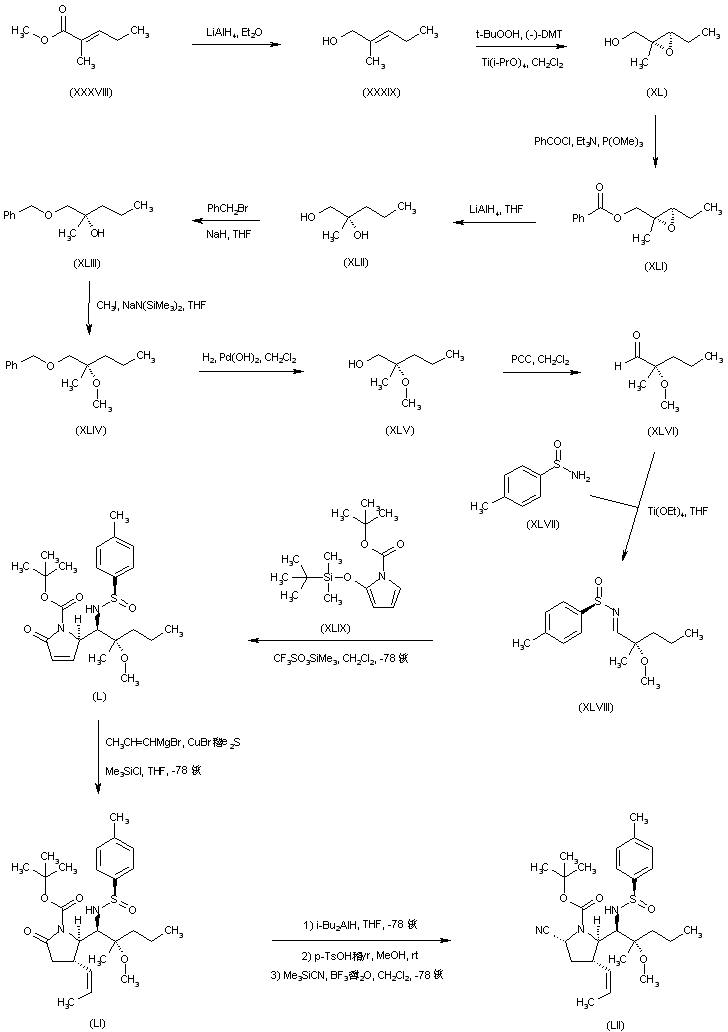 |
| 合成路线图解说明: In a different procedure, reduction of methyl 2-methyl-2-pentenoate (XXXVIII) with LiAlH4 afforded the allylic alcohol (XXXIX). Sharpless asymmetric epoxidation of (XXXIX) provided the chiral epoxy alcohol (XL), which was subsequently esterified with benzoyl chloride, yielding the epoxy benzoate (XLI). Reduction of (XLI) with LiAlH4 furnished diol (XLII). After protection of the primary alcohol of (XLII) as the benzyl ether (XLIII), its tertiary hydroxyl group was alkylated with iodomethane to give the methoxy derivative (XLIV). Hydrogenolysis of the benzyl ether group of (XLIV) over Pearlman's catalyst, followed by oxidation of the resultant alcohol (XLV) with pyridinium chlorochromate, led to aldehyde (XLVI). This was condensed with p-toluenesulfinamide (XLVII) in the presence of titanium ethoxide producing the sulfinimide (XLVIII). Diastereoselective condensation of N-Boc-2-(tert-butyldimethylsilyloxy)pyrrole (XLIX) with the chiral sulfinimide (XLVIII) gave rise to the pyrrolidone adduct (L). Copper-catalyzed conjugate addition of propenylmagnesium bromide to the unsaturated lactam (L) provided the (Z)-propenyl pyrrolidinone (LI). Introduction of a cyano group to give (LII) was accomplished by lactam reduction with DIBAL followed by treatment with MeOH and PPTS and cyanide addition as above. |
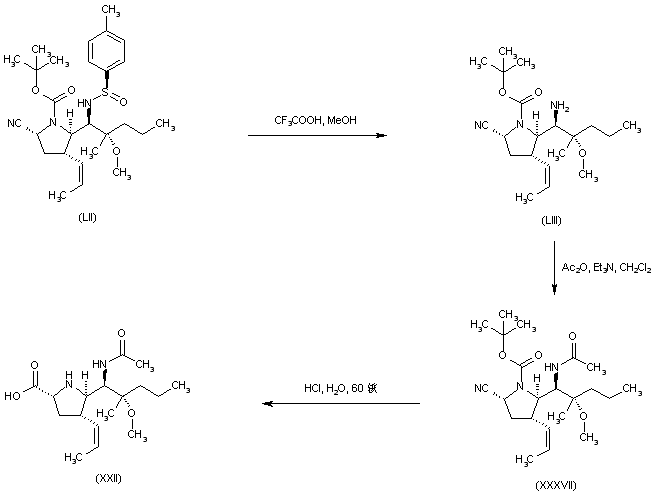 |
| 合成路线图解说明: Acidic cleavage of the chiral sulfinimide group of (LII) provided amine (LIII), which was acylated with Ac2O, yielding acetamide (XXXVII). Finally, hydrolysis and deprotection of (XXXVII) as in Scheme 30409701e gave rise to the title compound. |
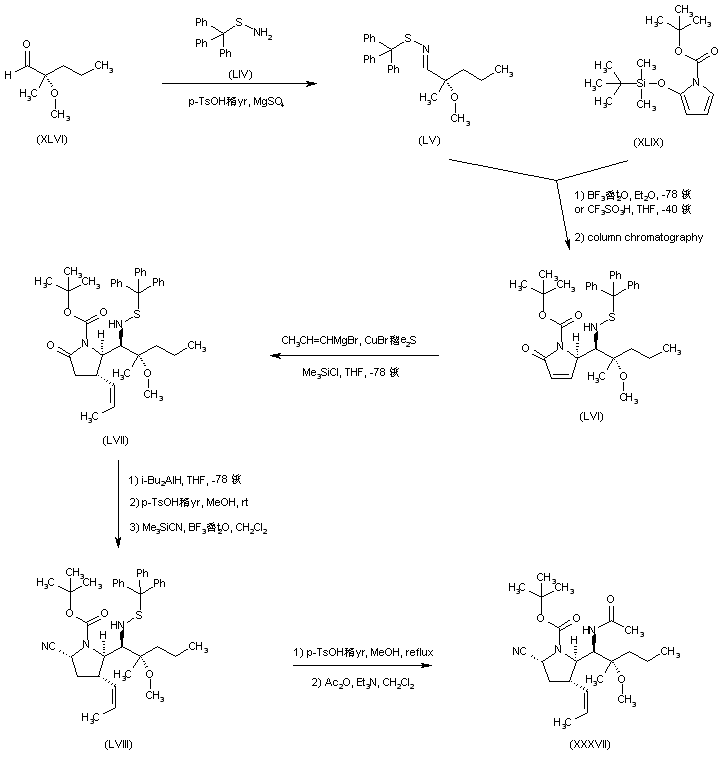 |
| 合成路线图解说明: In a related procedure, aldehyde (XLVI) was condensed with triphenylmethanesulfenamide (LIV), producing the S-trityl sulfenimine (LV). Aldol-type condensation of (LV) with the silyloxy pyrrole (XLIX) gave rise to a diastereoisomeric mixture of adducts from which the desired isomer (LVI) was isolated as the major compound. Diastereoselectivity was increased using triflic acid as the condensation catalyst. Conjugate addition to (LVI) of propenylmagnesium bromide in the presence of CuBr yielded (LVII). Conversion of pyrrolidinone (LVII) to the cyano pyrrolidine (LVIII) was accomplished by the same sequence as above. Then, acidic cleavage of the tritylsulfanyl group of (LVIII), followed by N-acetylation, furnished the intermediate (XXXVII), which was finally processed as in the preceding methods. |
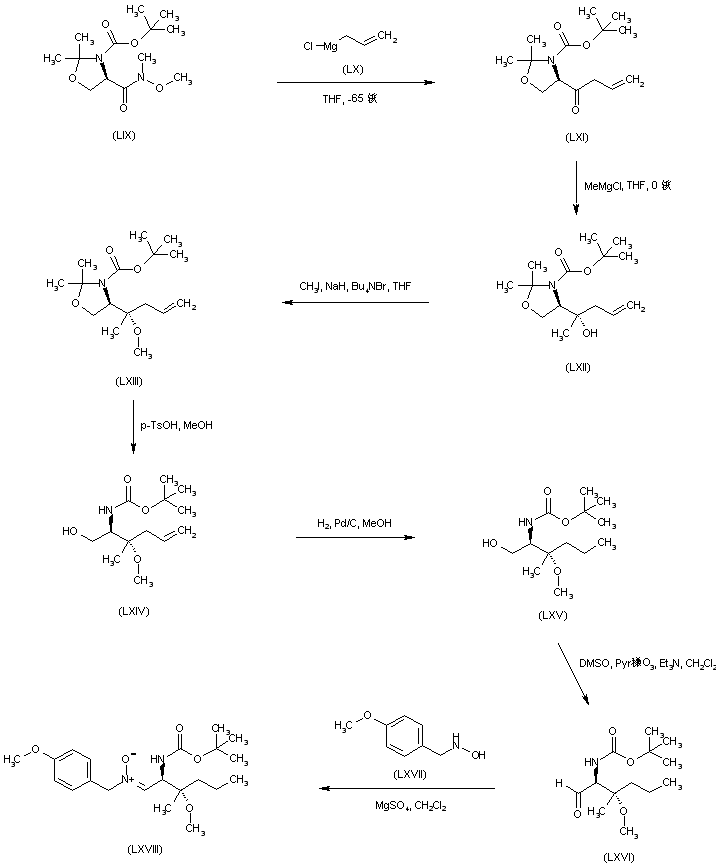 |
| 合成路线图解说明: In a different procedure, addition of the allyl Grignard reagent (LX) to the N-methoxy amide (LIX), provided ketone (LXI). Subsequent addition of methylmagnesium chloride to (LXI) led to the tertiary alcohol (LXII), which was converted to the methyl ether (LXIII) by alkylation with iodomethane and NaH. Acetonide (LXIII) hydrolysis with p-toluenesulfonic acid in MeOH gave the N-Boc amino alcohol (LXIV). The olefin double bond of (LXIV) was then hydrogenated over Pd/C to the propyl derivative (LXV). Oxidation of alcohol (LXV) under modified Swern conditions furnished aldehyde (LXVI). This was then condensed with p-methoxybenzyl hydroxylamine (LXVII), leading to the nitrone (LXVIII). |
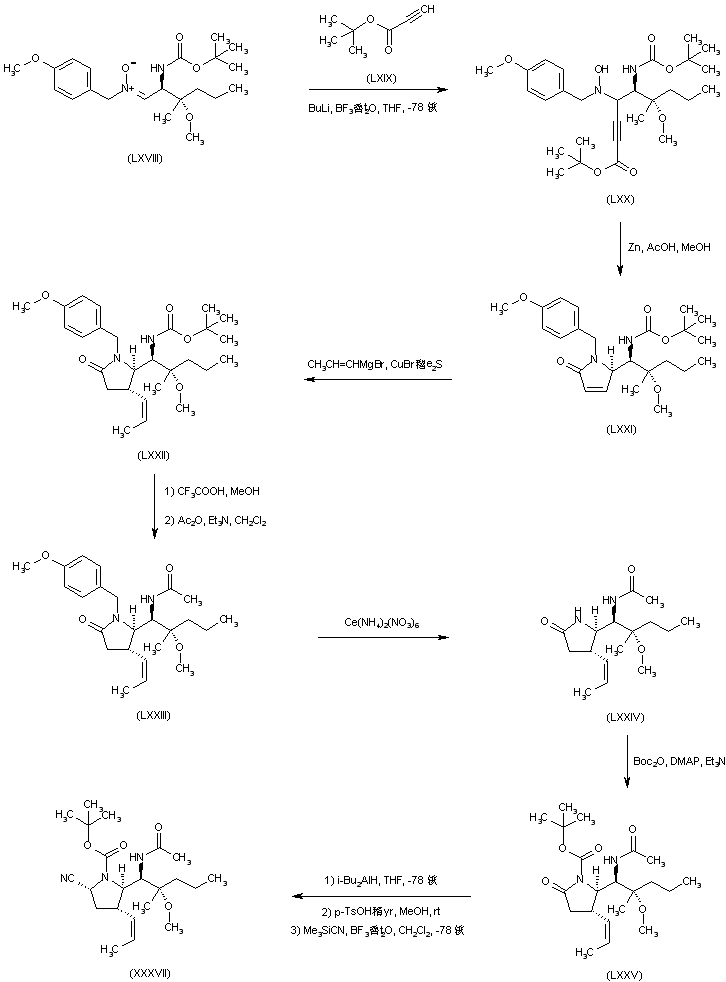 |
| 合成路线图解说明: Addition of the lithium anion of tert-butyl propiolate (LXIX) to nitrone (LXVIII) in the presence of BF3稥t2O gave adduct (LXX). Reduction of the hydroxylamine (LXX), with concomitant cyclization by treatment with Zn and HOAc, produced pyrrolidone (LXXI). The propenyl group of (LXXII) was introduced by conjugate addition of the corresponding organocuprate reagent to the unsaturated lactam (LXXI). Acidic Boc group cleavage in (LXXII), followed by acetylation of the resultant amine, afforded acetamide (LXXIII). Oxidative cleavage of the p-methoxybenzyl group of (LXXIII) using ammonium cerium nitrate gave pyrrolidone (LXXIV), which was further protected as the N-Boc derivative (LXXV). Introduction of the cyano group in (LXXV) as in the above methods led to the common precursor (XXXVII). |
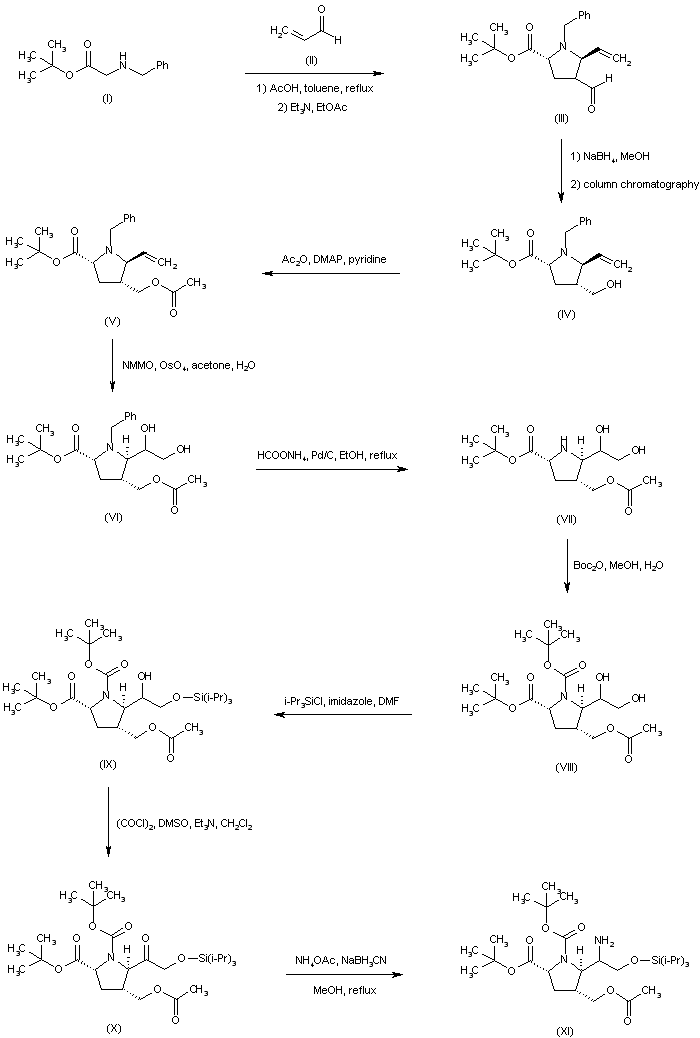 |
| 合成路线图解说明: The condensation between N-benzylglycine t-butyl ester (I) and acrolein (II) furnished pyrrolidine (III) as a mixture of epimers at C-4. The mixture of aldehydes was further equilibrated to an 8:1 ratio by stirring the crude product with Et3N in EtOAc. Aldehyde (III) reduction employing NaBH4 produced the corresponding mixture of alcohols, from which the desired isomer (IV) was isolated by column chromatography. After protection of the alcohol function of (IV) as the acetate ester (V), dihydroxylation of the vinyl group of (V) with N-methylmorpholine N-oxide in the presence of OsO4 provided diol (VI). The N-benzyl group of (VI) was then removed by transfer hydrogenolysis with ammonium formate and Pd/C to give the secondary amine (VII), which was further converted to the N-Boc derivative (VIII). Selective silylation of the primary hydroxyl group of (VIII) employing triisopropylsilyl chloride and imidazole afforded the silyl ether (IX). The secondary hydroxyl group of (IX) was then oxidized under Swern conditions to ketone (X). Reductive amination of (X) with ammonium acetate in the presence of NaBH3CN led to a mixture of epimeric amines (XI). |
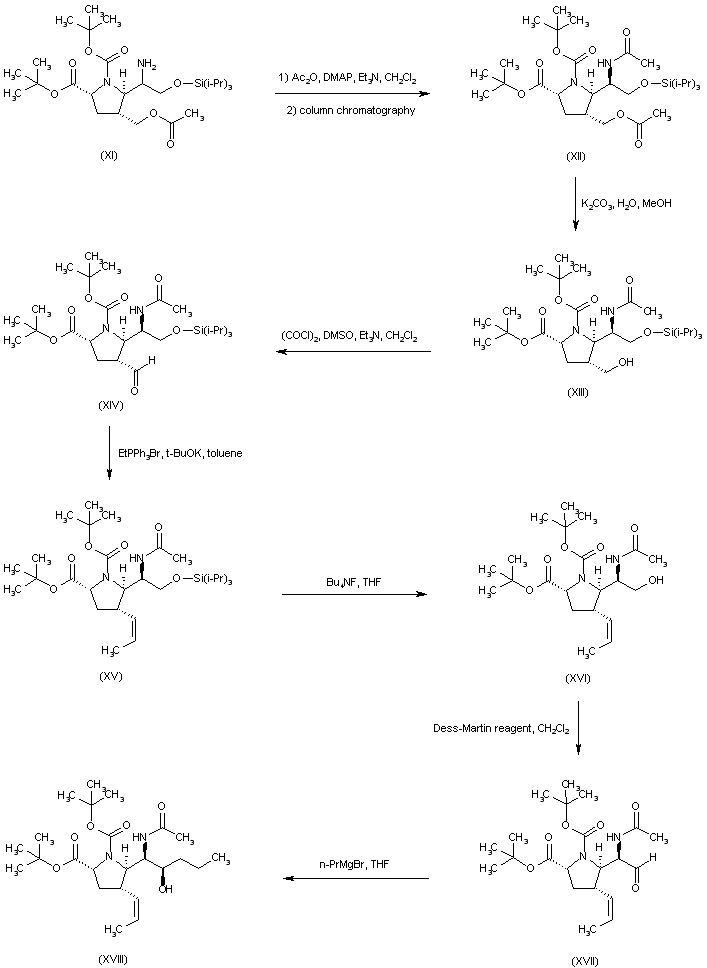 |
| 合成路线图解说明: The mixture of amines (XI) was converted to the respective acetamides, and the desired isomer (XII) was then isolated by column chromatography. Hydrolysis of the acetate ester function of (XII) provided alcohol (XIII), which was oxidized to aldehyde (XIV) under Swern conditions. Wittig reaction of aldehyde (XIV) with ethyl triphenylphosphonium bromide and potassium tert-butoxide led to the (Z)-olefin (XV). After removal of the O-silyl protecting group of (XV) by means of tetrabutylammonium fluoride, the resultant alcohol (XVI) was oxidized to aldehyde (XVII) employing the Dess-Martin periodinane reagent. Addition of propylmagnesium bromide to aldehyde (XVII) furnished the alcohol adduct (XVIII). |
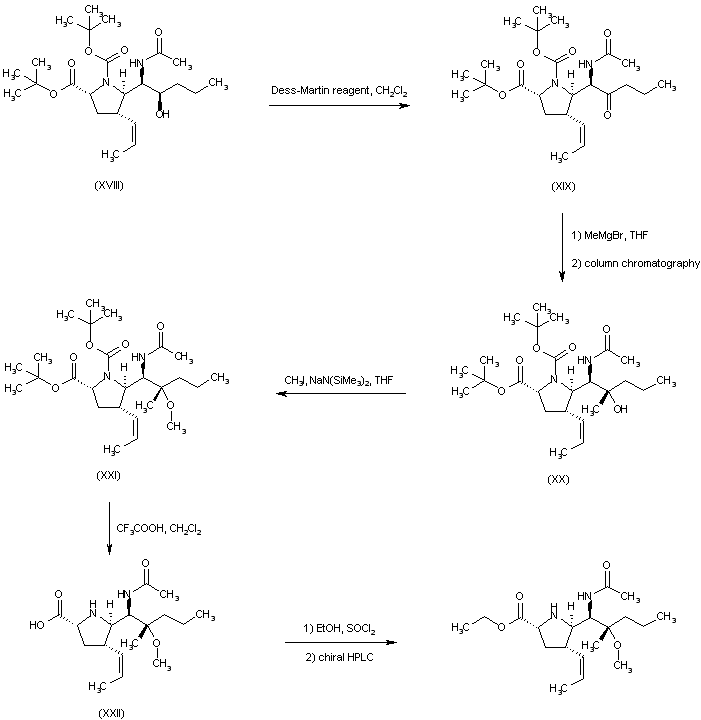 |
| 合成路线图解说明: Oxidation of alcohol (XVIII) employing the Dess-Martin periodinane reagent furnished ketone (XIX). Subsequent addition of MeMgBr to ketone (XIX) gave rise to a mixture of diastereoisomeric carbinols from which alcohol (XX) was isolated as the major isomer. The methyl ether (XXI) was prepared by alkylation of the sodium alkoxide of (XX) with iodomethane in THF. Trifluoroacetic acid-promoted cleavage of the tert-butyl ester and N-Boc protecting groups of (XXI) provided the carboxylic acid (XXII). The title ester enantiomer was then obtained by esterification of racemic (XXII) with EtOH and SOCl2, followed by resolution using chiral HPLC. |
 |
| 合成路线图解说明: The condensation between N-benzylglycine t-butyl ester (I) and acrolein (II) furnished pyrrolidine (III) as a mixture of epimers at C-4. The mixture of aldehydes (III) was further equilibrated to an 8:1 ratio by stirring the crude product with Et3N in EtOAc. Aldehyde (III) reduction employing NaBH4 produced the corresponding mixture of alcohols, from which the desired isomer (IV) was isolated by column chromatography. After protection of the alcohol function of (IV) as the acetate ester (V), dihydroxylation of the vinyl group of (V) with N-methylmorpholine N-oxide in the presence of OsO4 provided diol (VI). The N-benzyl group of (VI) was then removed by transfer hydrogenolysis with ammonium formate and Pd/C to give the secondary amine (VII), which was further converted to the N-Boc derivative (VIII). Selective silylation of the primary hydroxyl group of (VIII) employing triisopropylsilyl chloride and imidazole afforded the silyl ether (IX). The secondary hydroxyl group of (IX) was then oxidized under Swern conditions to ketone (X). Reductive amination of (X) with ammonium acetate in the presence of NaBH3CN led to a mixture of epimeric amines (XI). |
 |
| 合成路线图解说明: The mixture of amines (XI) was converted to the respective acetamides and the desired isomer (XII) was then isolated by column chromatography. Hydrolysis of the acetate ester function of (XII) provided alcohol (XIII), which was oxidized to aldehyde (XIV) under Swern conditions. Wittig reaction of aldehyde (XIV) with ethyl triphenylphosphonium bromide and potassium tert-butoxide led to the (Z)-olefin (XV). After removal of the O-silyl protecting group of (XV) by means of tetrabutylammonium fluoride, the resultant alcohol (XVI) was oxidized to aldehyde (XVII) employing the Dess-Martin periodinane reagent. Addition of propylmagnesium bromide to aldehyde (XVII) furnished the alcohol adduct (XVIII). |
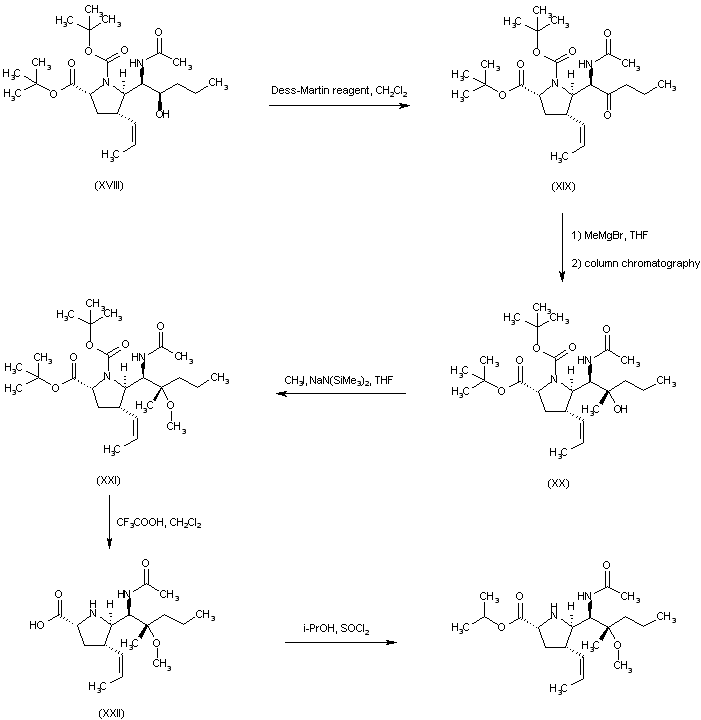 |
| 合成路线图解说明: Oxidation of alcohol (XVIII) employing the Dess-Martin periodinane reagent furnished ketone (XIX). Subsequent addition of methylmagnesium bromide to ketone (XIX) gave rise to a mixture of diastereoisomeric carbinols from which alcohol (XX) was isolated as the major isomer. The methyl ether (XXI) was prepared by alkylation of the sodium alkoxide of (XX) with iodomethane in THF. Trifluoroacetic acid-promoted cleavage of the tert-butyl ester and N-Boc protecting groups of (XXI) provided the carboxylic acid (XXII). The title isopropyl ester was then obtained by esterification of acid (XXII) with 2-propanol and SOCl2. |