| 参考文献No. | 53438 |
| 标题: | (1'S)-Hydroxyalkyloxapenem-3-carboxylic acids and their use as beta-lactamase inhibitors |
| 作者: | Pfaendler, H.R. (Eli Lilly and Company) |
| 来源: | DE 4142423; EP 0548790 |
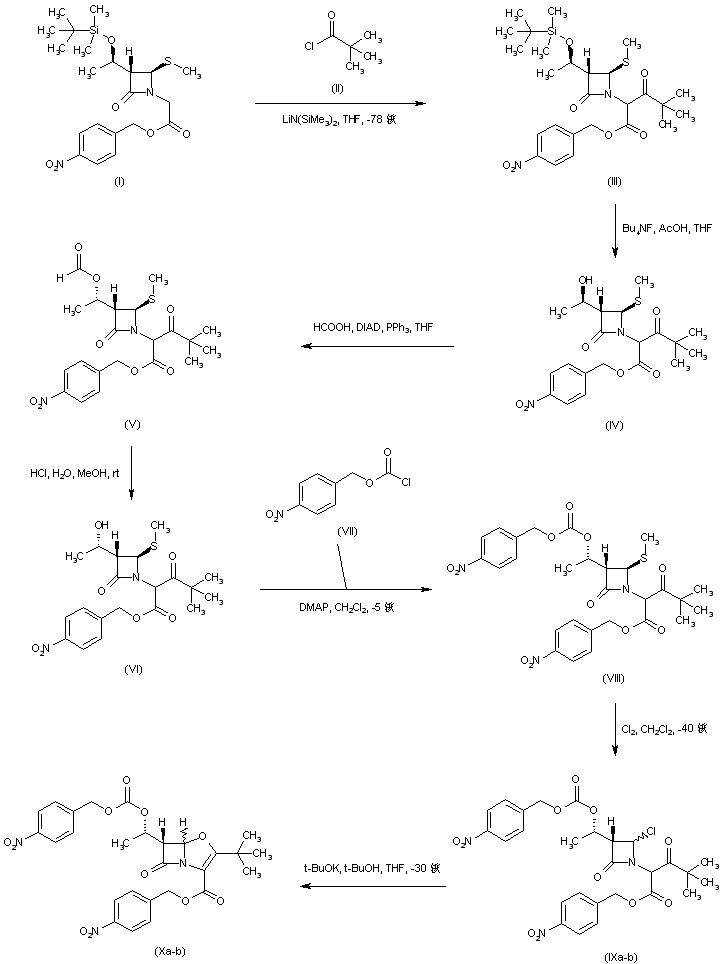 |
| 合成路线图解说明: The title compound has been prepared starting from the known azetidinone (I). The lithium enolate of ester (I) was acylated with pivaloyl chloride (II) at low temperature to afford keto ester (III). The silyl protecting group of (III) was then removed by treatment with tetrabutylammonium fluoride, yielding alcohol (IV). Inversion of the configuration of alcohol (IV) was accomplished by Mitsunobu coupling with formic acid to produce the formate ester (V), which was subsequently hydrolyzed to the desired alcohol (VI) under acidic conditions. Protection of alcohol (VI) with p-nitrobenzyl chloroformate (VII) gave carbonate (VIII). Chlorination of (VIII) with concomitant oxidative cleavage of the methylthio group at -40 C led to the chloroazetidine (IX). Cyclization of (IX) to the key oxapenem bicyclic system (X) was then performed by treatment with potassium tert-butoxide in cold t-BuOH/THF. |
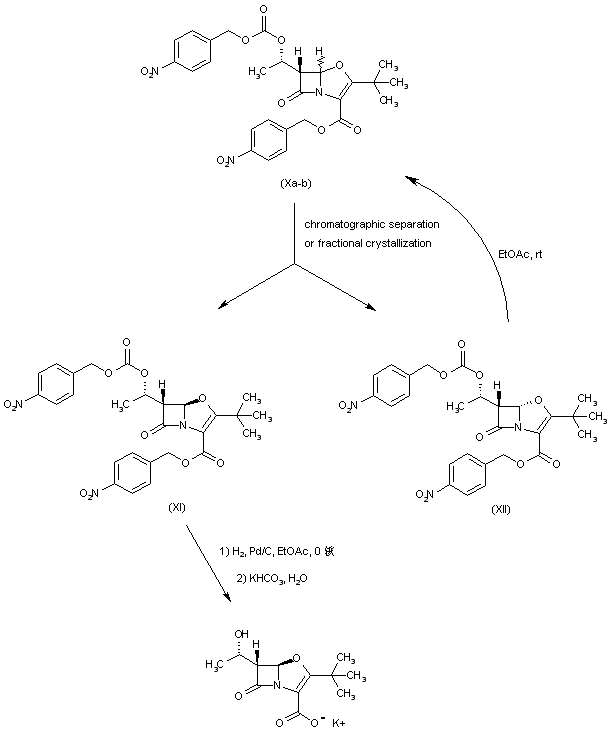 |
| 合成路线图解说明: The cis/trans mixture of bicyclic compounds (X) was separated either by column chromatography at low temperature or by fractional crystallization. The desired isomer (XI) was then subjected to catalytic hydrogenolysis of the p-nitrobenzyl ester groups, and the resultant carboxylic acid was then converted to the title potassium salt upon treatment with aqueous potassium bicarbonate. Recycling of the undesired isomer (XII) was possible because of slow isomerization of an EtOAc solution of (XII) at room temperature. |
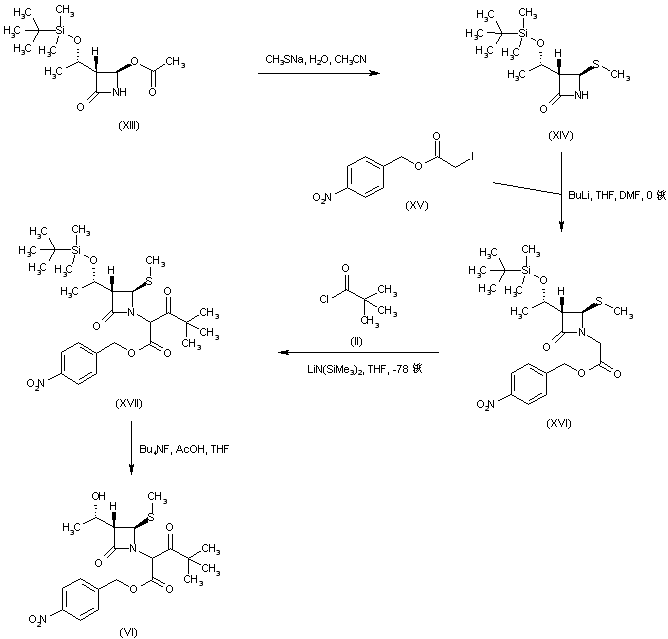 |
| 合成路线图解说明: In an alternative route, intermediate (VI) was prepared from the previously reported azetidinone (XIII), possessing the adequate alcohol group configuration. Displacement of the acetoxy group of (XIII) with sodium methanethiolate gave sulfide (XIV). The azetidinone N was then alkylated with p-nitrobenzyl iodoacetate (XV) to afford azetidineacetate (XVI). Acylation of the lithium enolate of ester (XVI) by pivaloyl chloride (II) provided keto ester (XVII). Intermediate (VI) was then obtained by desilylation of (XVII) with tetrabutylammonium fluoride. |