| 参考文献No. | 32047 |
| 标题: | Indolinone cpds. for the treatment of disease |
| 作者: | Tang, P.C.; Sun, L.; McMahon, G. (Sugen, Inc.) |
| 来源: | EP 0769947; JP 2000026412; US 5780496; US 5792783; US 5880141; US 5883113; US 5883116; WO 9640116 |
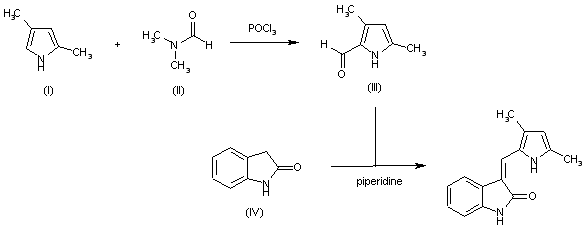 |
| 合成路线图解说明: Vilsmeier reaction between 2,4-dimethylpyrrole (I) and dimethylformamide (II) by means of phosphorous oxychloride in dichloroethane provides carbaldehyde (III), which is then condensed with 2-indolinone (IV) in ethanol in the presence of piperidine. |
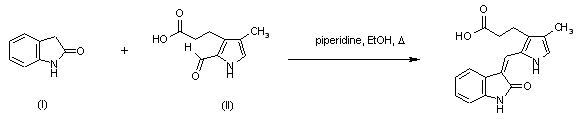 |
| 合成路线图解说明: Title compound was prepared by condensation of oxindole (I) and formylpyrrole (II) in the presence of a catalytic amount of piperidine in refluxing EtOH. |
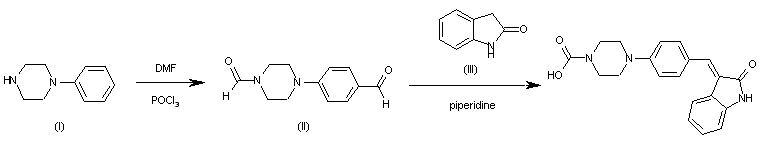 |
| 合成路线图解说明: The reaction of 1-phenylpiperazine (I) with DMF and POCl3 in dichloroethane gives 4-(4-formylpiperazin-1-yl)benzaldehyde (II), which is condensed with indolin-2-one (III) by means of piperidine in refluxing ethanol. |
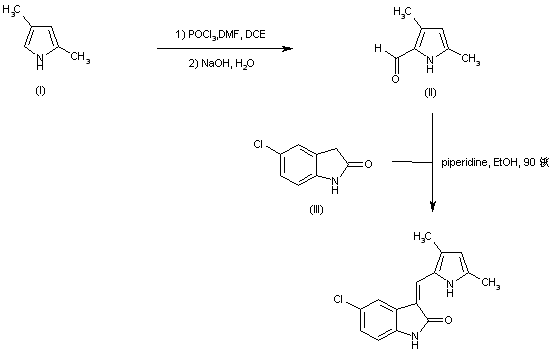 |
| 合成路线图解说明: Aldehyde (II) was prepared by Vilsmeier-Haack formylation of 2,4-dimethylpyrrole (I). Subsequent condensation of the pyrrole aldehyde (II) with 5-chlorooxindole (III) in the presence of piperidine furnished the title compound. |