| 参考文献No. | 644093 |
| 标题: | Synthesis and biological evaluation of a series of novel N- or O-fluoroalkyl derivatives of tropane: Potential positron emission tomography (PET) imaging agents for the dopamine transporter |
| 作者: | Gu, X.-H.; Zong, R.; Kula, N.S.; Baldessarini, R.J.; Neumeyer, J.L. |
| 来源: | Bioorg Med Chem Lett 2001,11(23),3049 |
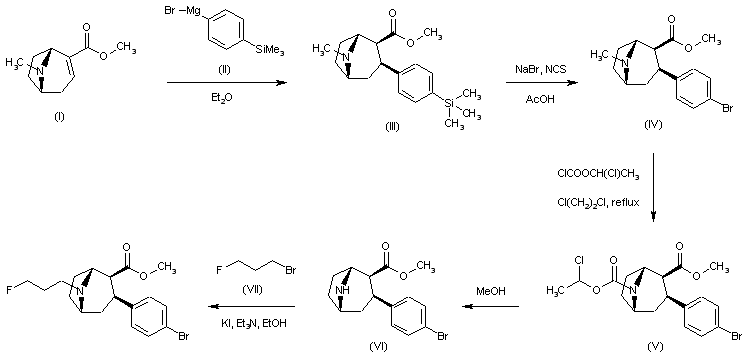 |
| 合成路线图解说明: Conjugate addition of 4-(trimethylsilyl)phenylmagnesium bromide (II) to anhydroecgonine methyl ester (I) afforded regioselectively the 2-beta-carbomethoxy-3-beta-aryl tropane (III). Bromodesilylation of (III) using NaBr and N-chlorosuccinimide gave the bromophenyl derivative (IV). Amine demethylation was accomplished by reaction of (IV) with 1-chloroethyl chloroformate, followed by hydrolysis and decarboxylation of the intermediate carbamate (V) upon treatment with methanol. The resulting secondary amine (VI) was then alkylated with 3-fluoropropyl bromide (VII) to furnish the title fluoropropyl amine. |
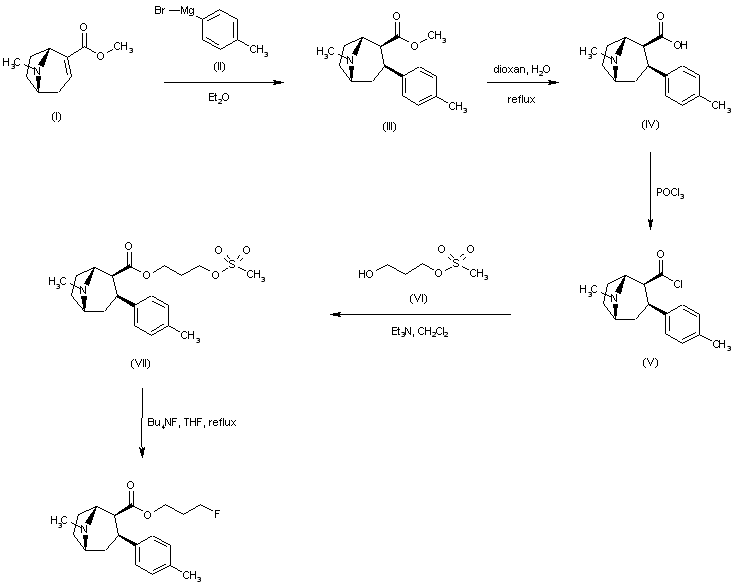 |
| 合成路线图解说明: Conjugate addition of 4-tolylmagnesium bromide (II) to anhydroecgonine methyl ester (I) afforded regioselectively the 2-beta-carbomethoxy-3-beta-aryl tropane (III). Hydrolysis of the ester function of (III) in aqueous dioxan gave the corresponding acid (IV). Treatment of (IV) with POCl3 yielded the acid chloride (V), which was condensed with 1,3-propanediol monomesylate (VI), yielding ester (VII). Finally, nucleophilic displacement of the mesylate group of (VII) with tetrabutylammonium fluoride in refluxing THF afforded the title fluoropropyl ester. |
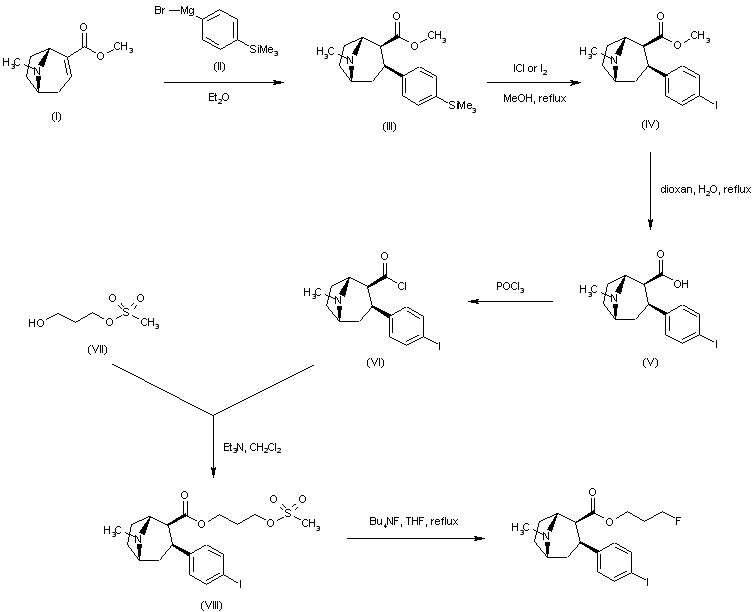 |
| 合成路线图解说明: Conjugate addition of 4-(trimethylsilyl)phenylmagnesium bromide (II) to anhydroecgonine methyl ester (I) afforded regioselectively the 2-beta-carbomethoxy-3-beta-aryl tropane (III). Iododesilylation of (III) using ICl or iodine in the presence of AgBF4 gave the iodophenyl derivative (IV). Hydrolysis of the ester function of (IV) in aqueous dioxan yielded the corresponding acid (V). Treatment of (V) with POCl3 provided the acid chloride (VI), which was condensed with 1,3-propanediol monomesylate (VII) to yield ester (VIII). Finally, nucleophilic displacement of the mesylate group of (VIII) with tetrabutylammonium fluoride in refluxing THF afforded the title fluoropropyl ester. |
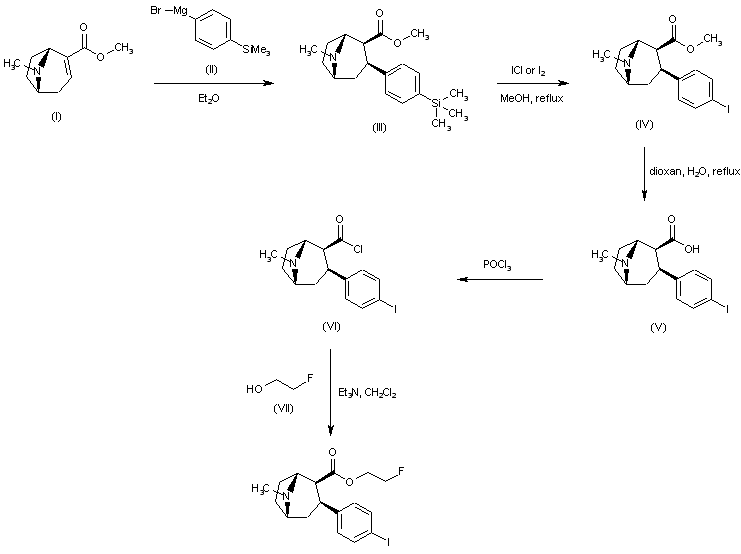 |
| 合成路线图解说明: Conjugate addition of 4-(trimethylsilyl)phenylmagnesium bromide (II) to anhydroecgonine methyl ester (I) afforded regioselectively the 2-beta-carbomethoxy-3-beta-aryl tropane (III). Iododesilylation of (III) using ICl or iodine in the presence of AgBF4 gave the iodophenyl derivative (IV). Hydrolysis of the ester function of (IV) in aqueous dioxan yielded the corresponding acid (V). Treatment of (V) with POCl3 provided the acid chloride (VI), which was finally condensed with 2-fluoroethanol (VII) to yield the title fluoroethyl ester. |