| 参考文献No. | 635123 |
| 标题: | Synthesis and in vitro evaluation of a series of diketopiperazine inhibitors of plasminogen activator inhibitor-1 |
| 作者: | Folkes, A.; Roe, M.B.; Sohal, S.; Golec, J.; Faint, R.; Brooks, T.; Charlton, P. |
| 来源: | Bioorg Med Chem Lett 2001,11(19),2589 |
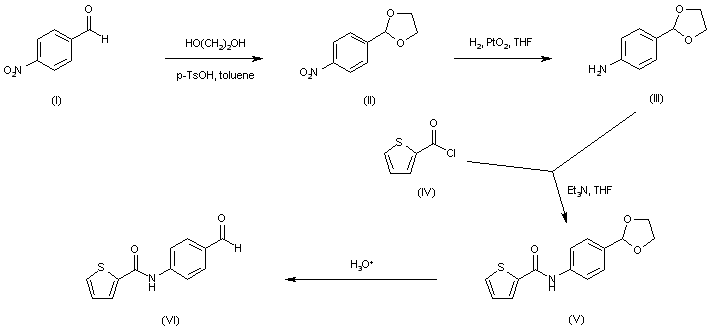 |
| 合成路线图解说明: The precursor aldehyde (VI) was prepared as follows. 4-Nitrobenzaldehyde (I) was protected as the ethylene acetal (II) and subsequently reduced by catalytic hydrogenation in the presence of PtO2 to furnish aniline (III). Acylation of (III) with 2-thiophenecarbonyl chloride (IV) provided amide (V). The ethylene acetal group of (V) was then hydrolyzed to the target aldehyde (VI) under acidic conditions. |
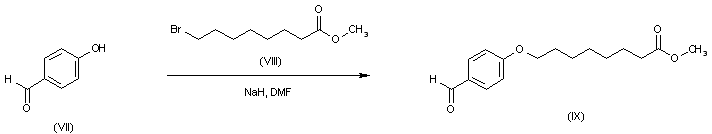 |
| 合成路线图解说明: The intermediate aldehyde (IX) was synthesized by alkylation of the sodium salt of 4-hydroxybenzaldehyde (VII) with methyl 8-bromooctanoate (VIII). |
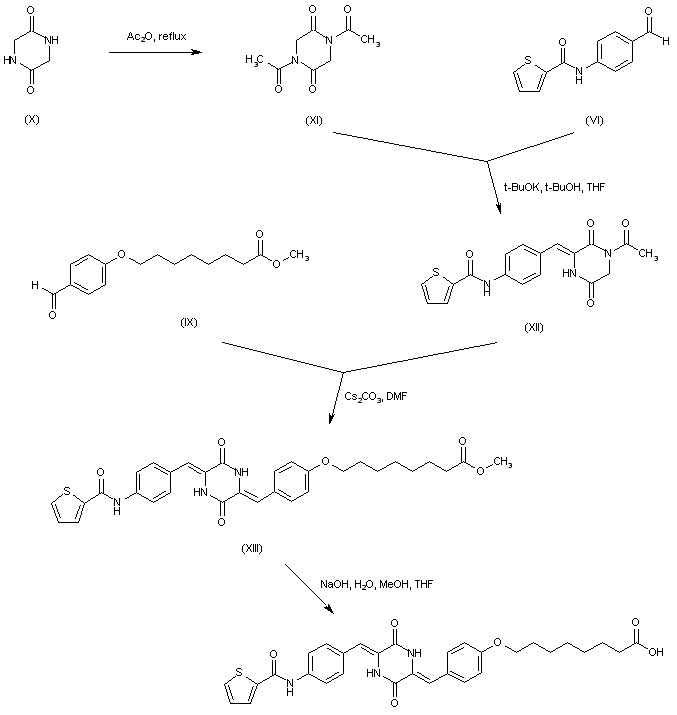 |
| 合成路线图解说明: Dioxopiperazine (X) was diacetylated in refluxing acetic anhydride to yield (XI). Aldol condensation of (XI) with the precursor aldehyde (VI) using potassium tert-butoxide in THF gave the mono-arylidene piperazine (XII). Further condensation of (XII) with the intermediate aldehyde (IX) in the presence of Cs2CO3 in DMF furnished the deacetylated bis-arylidene piperazine (XIII). The methyl ester group of (XIII) was finally hydrolyzed to the title carboxylic acid using NaOH in H2O/MeOH/THF. |