| 参考文献No. | 41934 |
| 标题: | Bispiperidines as antithrombotic agents |
| 作者: | Lesur, B.; Henry, M.; Yue, C.; Giboulot, T. (Laboratoires L. Lafon) |
| 来源: | EP 1098878; FR 2781223; JP 2002520393; US 6333338; WO 0003986 |
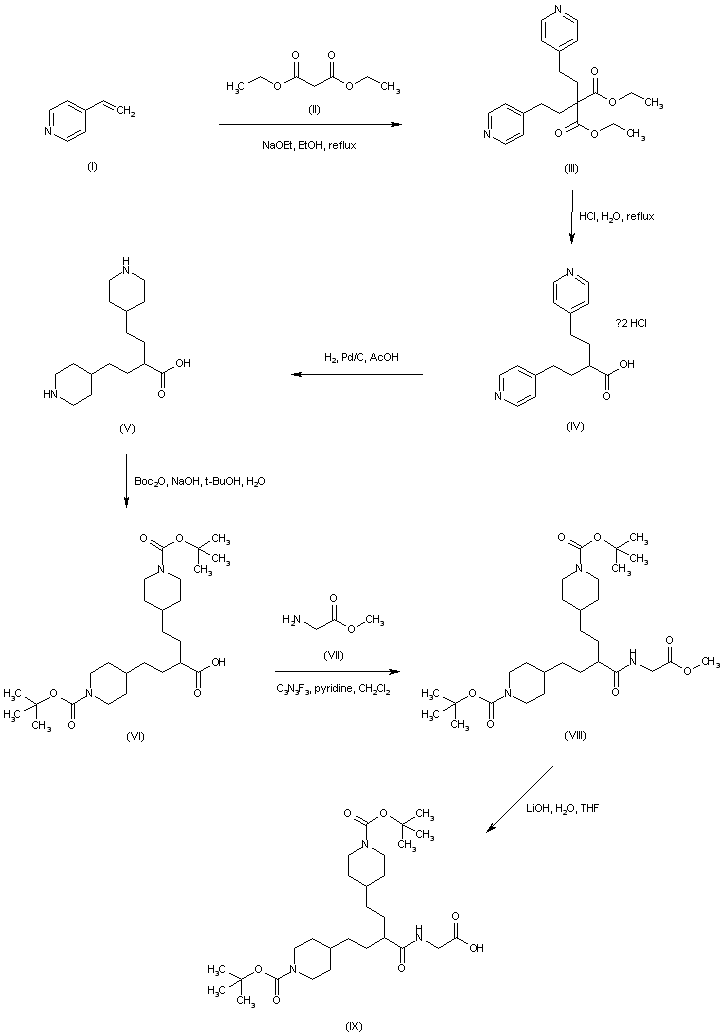 |
| 合成路线图解说明: Condensation of 4-vinylpyridine (I) with diethyl malonate (II) affords the bis(pyridylethyl)malonate (III) which, upon acidic hydrolysis and decarboxylation leads to mono-acid (IV). Catalytic hydrogenation of the pyridine rings of (IV) in the presence of Pd/C furnishes the corresponding piperidinyl compound (V), which is further protected as the N-Boc derivative (VI) employing di-t-butyl dicarbonate. Acid (VI) is coupled to glycine methyl ester (VII) via activation with cyanuric fluoride to yield amide (VIII). Then, alkaline hydrolysis of the methyl ester group provides carboxylic acid (IX). |
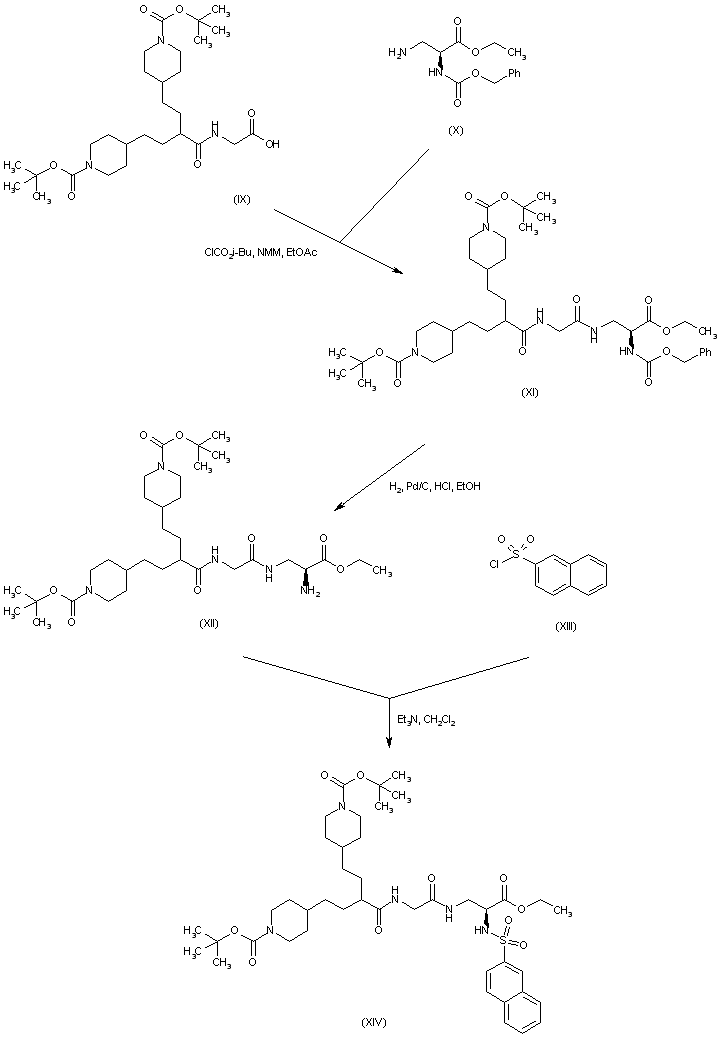 |
| 合成路线图解说明: After activation of acid (IX) as the mixed anhydride with isobutyl chloroformate, coupling with the mono-protected diaminopropionate (X) leads to amide (XI). Subsequent removal of the N-carbobenzoxy group by catalytic hydrogenolysis gives amino ester (XII). This is then acylated by 2-naphthalenesulfonyl chloride (XIII), yielding sulfonamide (XIV). |
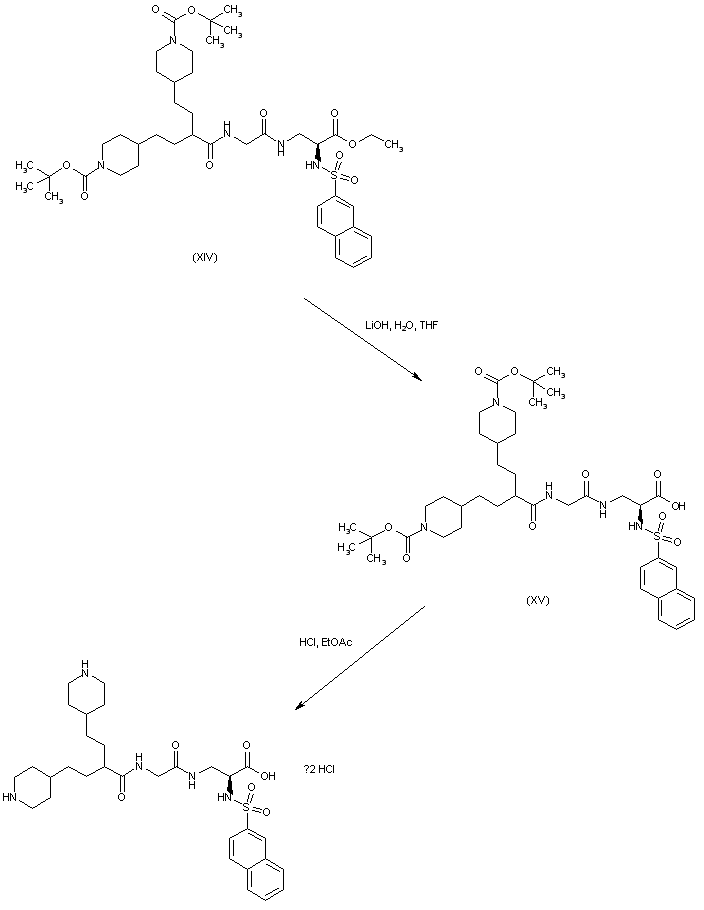 |
| 合成路线图解说明: Hydrolysis of ethyl ester (XIV) employing LiOH affords the carboxylic acid (XV). The N-Boc protecting groups are finally removed by treatment with HCl in EtOAc to provide the title compound. |