| 参考文献No. | 609655 |
| 标题: | Synthesis of bifunctional cationic compound for gene delivery |
| 作者: | Ren, T.; et al. |
| 来源: | Tetrahedron Lett 2001,42(6),1007 |
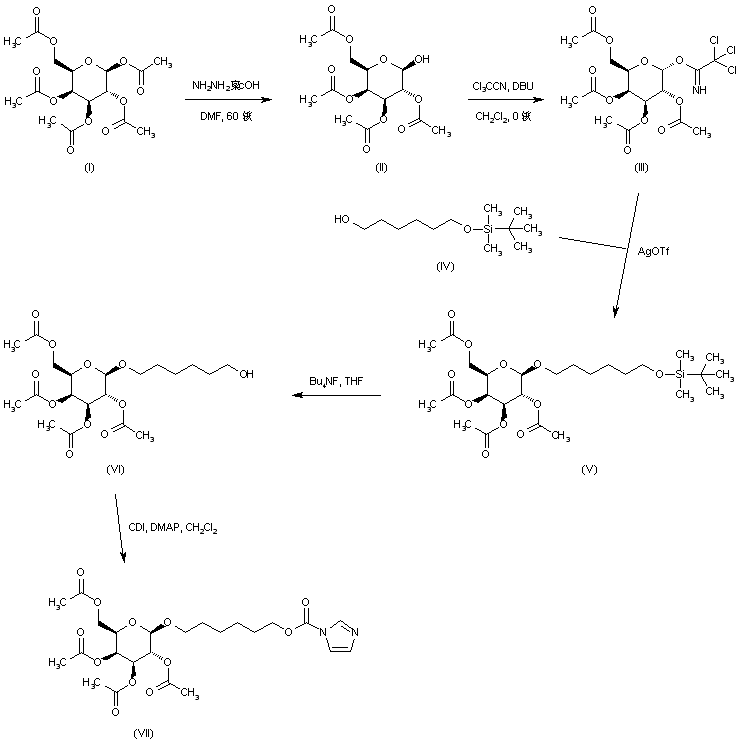 |
| 合成路线图解说明: Selective hydrolysis of the anomeric acetyl group from beta-D-galactose pentaacetate (I) was achieved by treatment with hydrazine acetate. The resulting hemiacetal (II) was then condensed with trichloroacetonitrile to furnish imidate (III). Glycosylation of the mono-silylated diol (IV) with imidate (III) in the presence of silver triflate provided the beta-glycoside derivative (V). After desilylation of (V) with tetrabutylammonium fluoride, the free alcohol (VI) was activated with carbonyl diimidazole to afford the imidazolide (VII). |
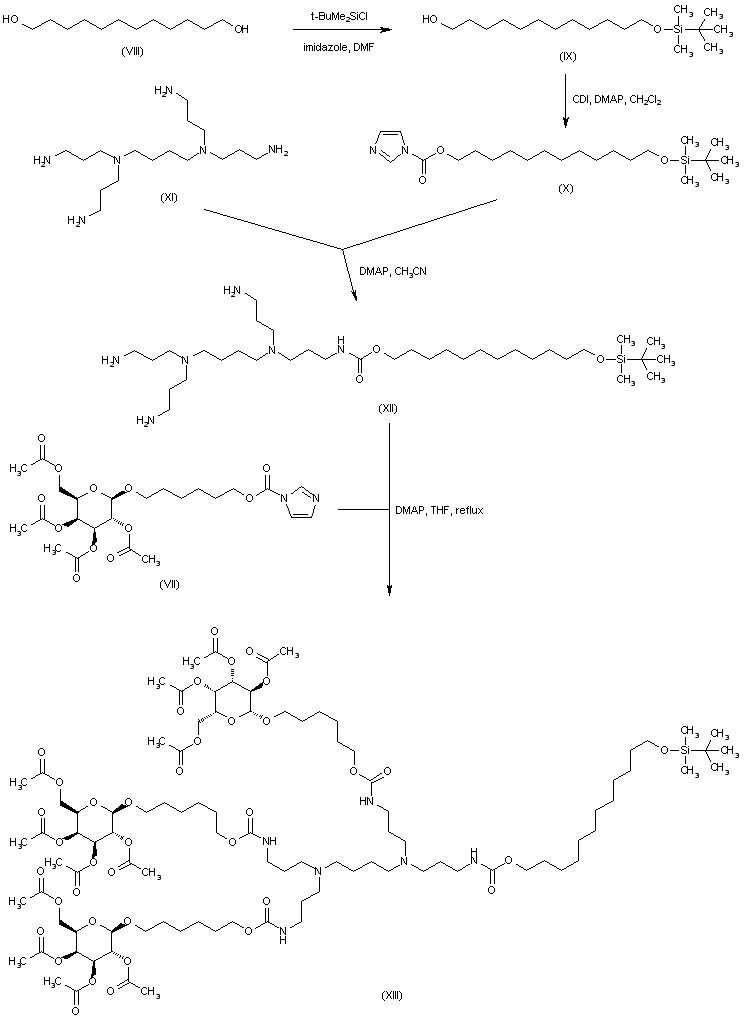 |
| 合成路线图解说明: 1,12-Dodecanediol (VIII) was monosilylated with tert-butyl dimethylsilyl chloride and the resultant silyl alcohol (IX) was further activated as the imidazolide (X). Condensation of (X) with the polyamine (XI) produced carbamate (XII). The remaining primary amino groups were then acylated with the imidazolide (VII), yielding the tetracarbamate (XIII). |
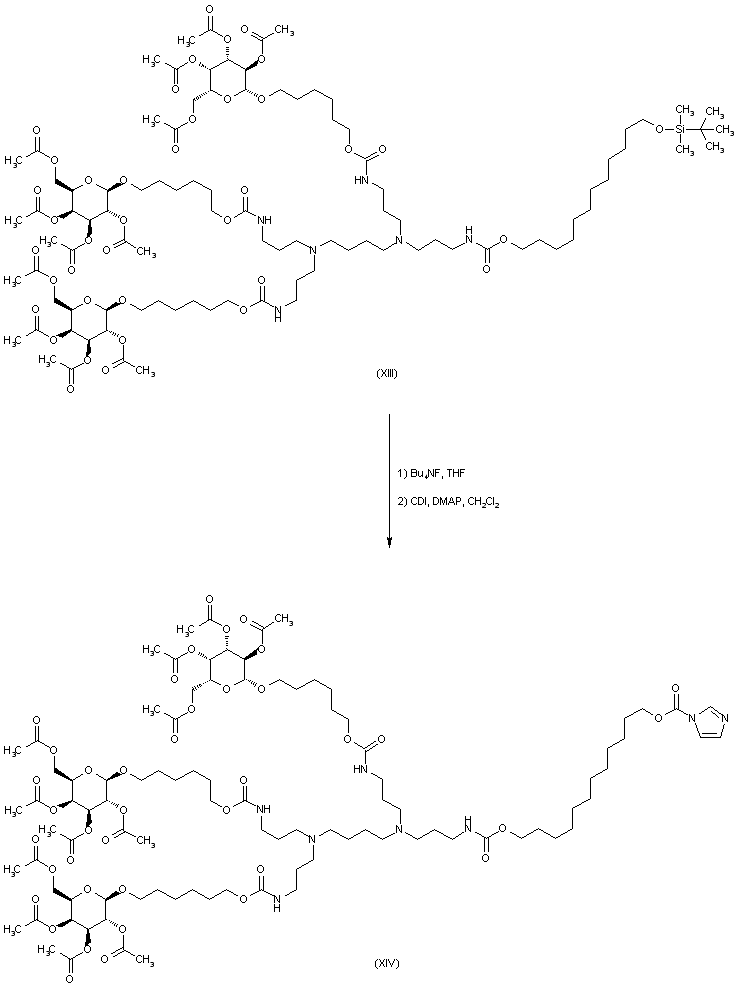 |
| 合成路线图解说明: After desilylation of (XIII) with tetrabutylammonium fluoride, the free alcohol was activated by a new treatment with CDI, producing imidazolide (XIV). |
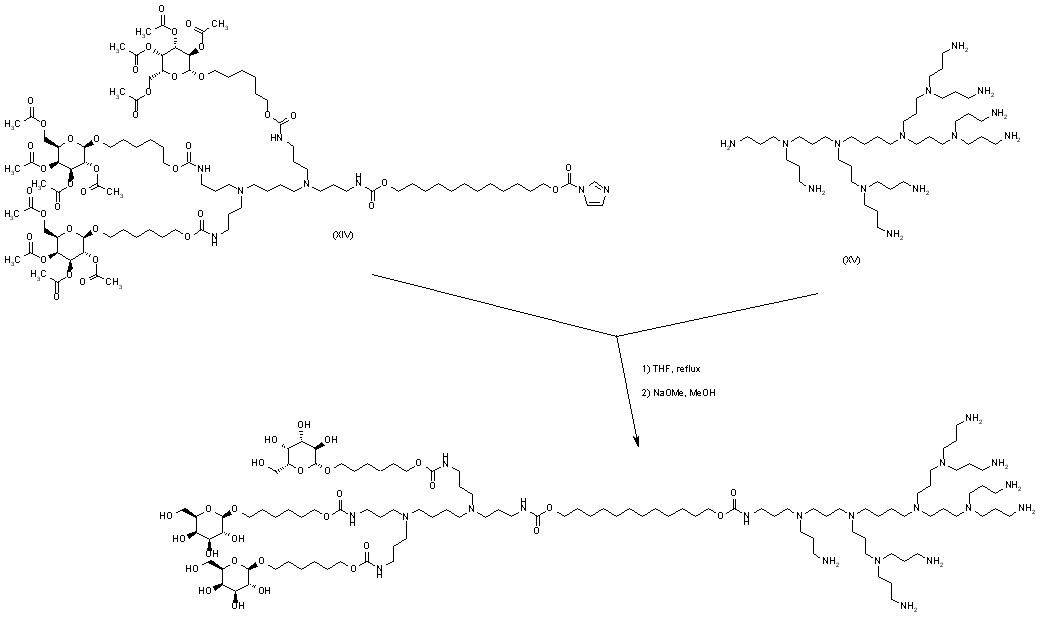 |
| 合成路线图解说明: Imidazolide (XIV) was then condensed with polyamine (XV) to give the corresponding carbamate. Final alcoholysis of the acetate ester groups with methanolic sodium methoxide furnished the title compound. |