| 参考文献No. | 44492 |
| 标题: | Immunological adjuvant cpd. |
| 作者: | Rossignol, D.P.; Ishizaka, S.T.; Nault, A.; Lewis, M.; Rose, J.; McGuiness, P.; Hawkins, L.D. (Eisai Co., Ltd.) |
| 来源: | EP 1147117; US 6290973; WO 0044758 |
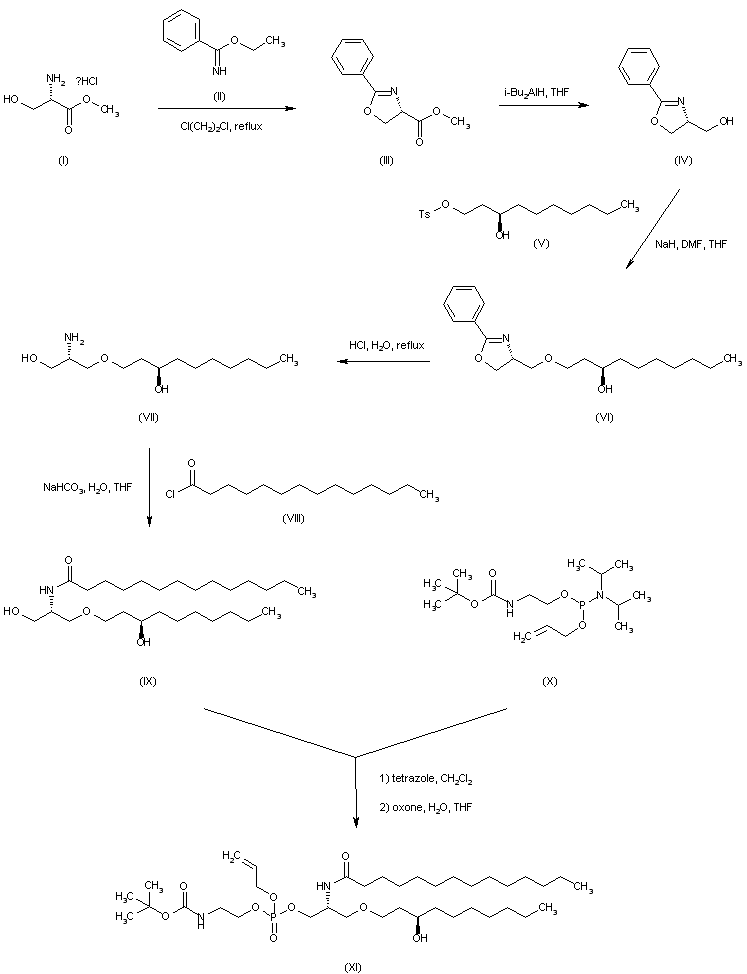 |
| 合成路线图解说明: L-Serine methyl ester (I) is condensed with ethyl benzimidate (II) to produce the oxazoline carboxylate (III). Subsequent reduction of ester (III) employing DIBAL in THF affords alcohol (IV). The sodium alkoxide of (IV) is alkylated with (R)-3-hydroxy-1-decyl p-toluenesulfonate (V) yielding ether (VI). Then acidic hydrolysis of the cyclic imidate (VI) furnishes amino diol (VII). Acylation of (VII) with myristoyl chloride (VIII) provides amide (IX). Phosphitylation of the primary hydroxyl of (IX) with phosphoramidite (X) followed by oxidation with oxone gives rise to the phosphate ester (XI). |
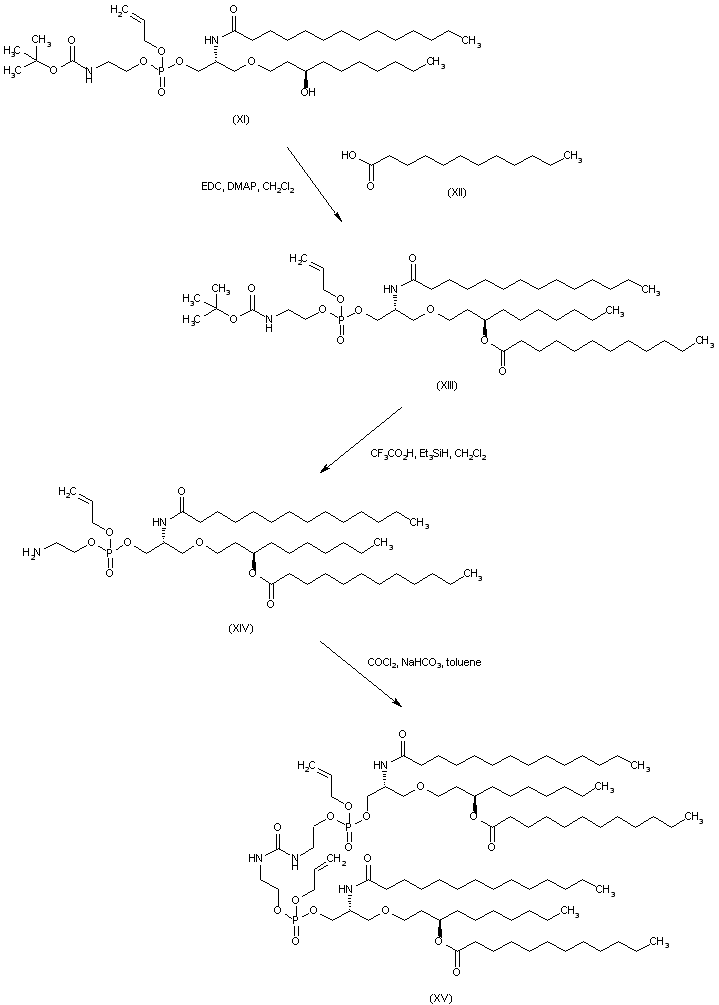 |
| 合成路线图解说明: Esterification of (XI) with dodecanoic acid (XII) in the presence of EDC/DMAP leads to ester (XIII). The N-Boc protecting group of (XIII) is subsequently cleaved under acidic conditions to afford amine (XIV). Then, condensation of two equivalents of amine (XIV) with phosgene in the presence of NaHCO3 produces urea (XV). |
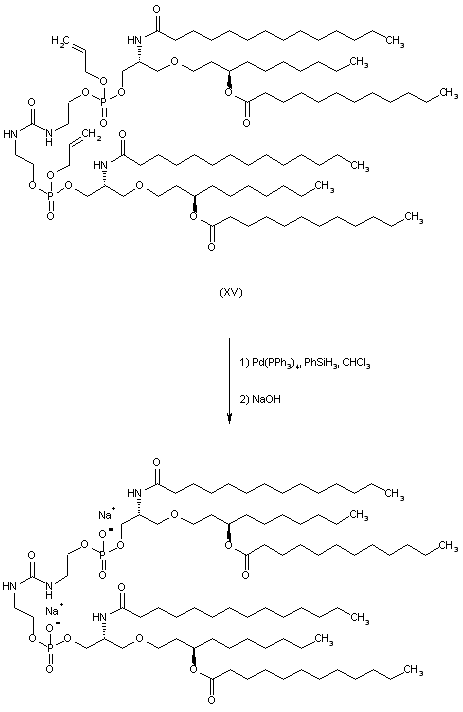 |
| 合成路线图解说明: The allyl phosphate ester groups of (XV) are removed by treatment with Pd(PPh3)4 in the presence of phenylsilane. The resultant phosphoric acid derivative is finally isolated as the title disodium salt by dissolution in aqueous NaOH followed by lyophilization. |
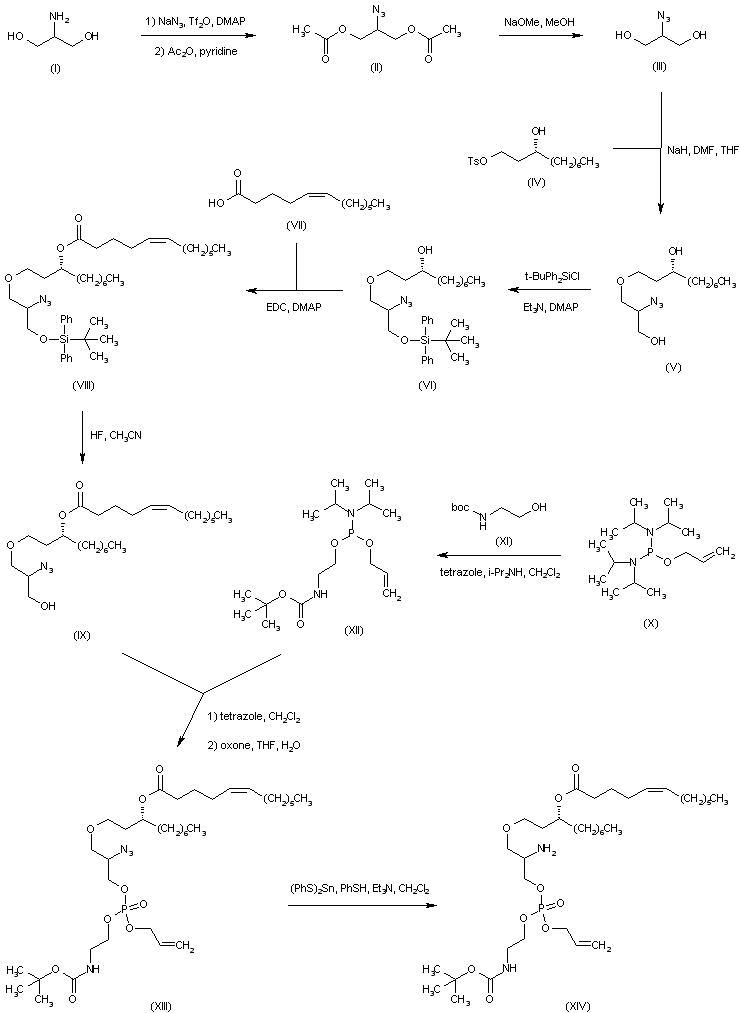 |
| 合成路线图解说明: 2-Amino-1,3-propanediol (I) was reacted with in situ generated trifluoromethanesulfonyl azide, and the resulting 2-azido compound was purified by chromatography after conversion to the diacetate ester (II). Deacetylation of (II) with sodium methoxide gave 2-azido-1,3-propanediol (III), which was condensed with tosylate (IV) in the presence of NaH to produce the dihydroxyether (V). The primary alcohol group of (V) was then protected as the mono-silyl derivative (VI) employing tert-butyldiphenylsilyl chloride. After acylation of the free hydroxyl group of (VI) with the unsaturated acid (VII), the resulting ester (VIII) was desilylated by using HF in acetonitrile to yield alcohol (IX). The phosphorylating reagent (XII) was prepared by treatment of allyl phosphorodiamidite (X) with N-Boc-ethanolamine (XI). Phosphitylation of alcohol (IX) with phosphoramidite (XII), followed by oxidation of the intermediate phosphite ester with oxone , furnished phosphate (XIII). The azido group of (XIII) was then reduced to the corresponding amine (XIV) by employing (PhS)2Sn. |
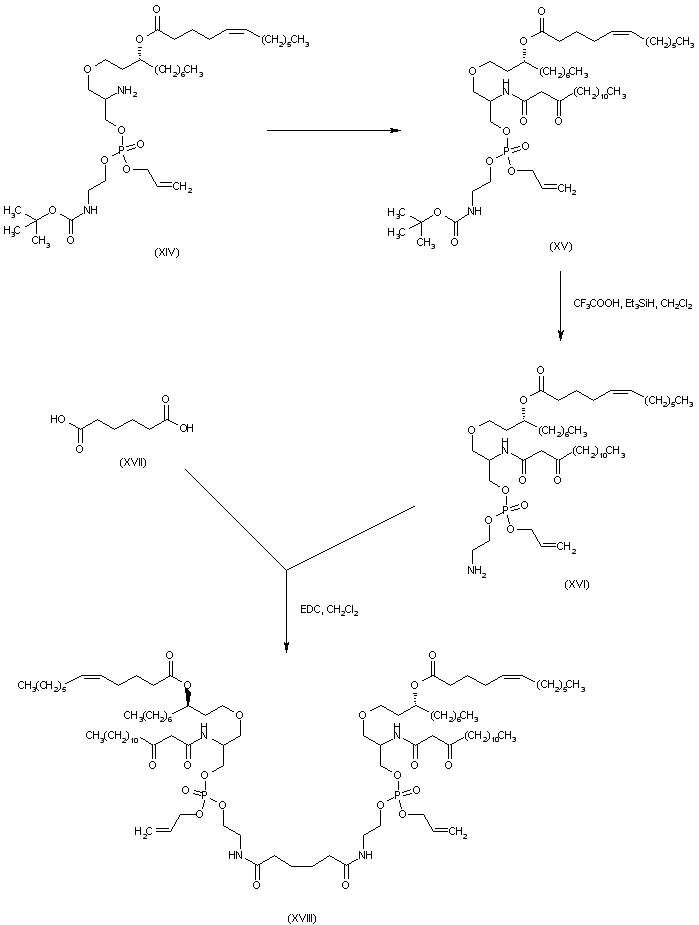 |
| 合成路线图解说明: After conversion of amine (XIV) to the ketoamide (XV), the N-Boc group was cleaved with trifluoroacetic acid to provide the primary amine (XVI). Condensation of adipic acid (XVII) with amine (XVI) in the presence of EDC gave rise to the diamide (XVIII). |
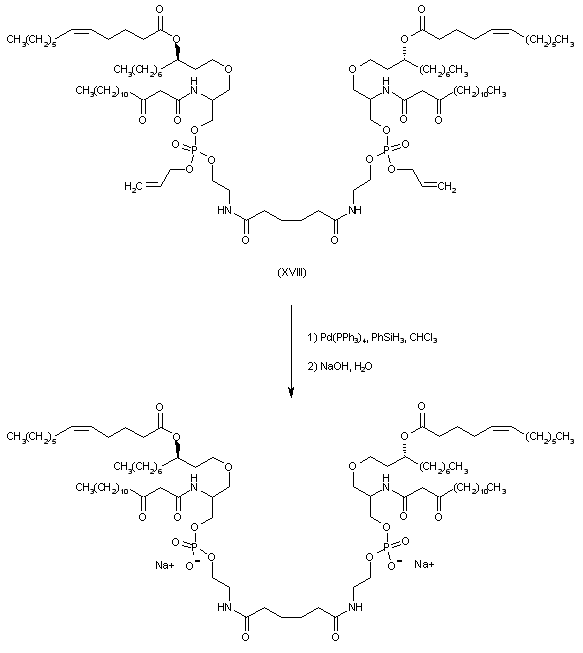 |
| 合成路线图解说明: The allyl phosphate groups were then deprotected by using phenylsilane and Pd catalyst, and the resulting diphosphoric acid was finally isolated as the corresponding disodium salt by lyophilization of its solution in aqueous NaOH. |