| 参考文献No. | 623952 |
| 标题: | Total synthesis of dendroamide A, a novel cyclic peptide that reverses multiple drug resistance |
| 作者: | Xia, Z.; Smith, C.D. |
| 来源: | J Org Chem 2001,66(10),3459 |
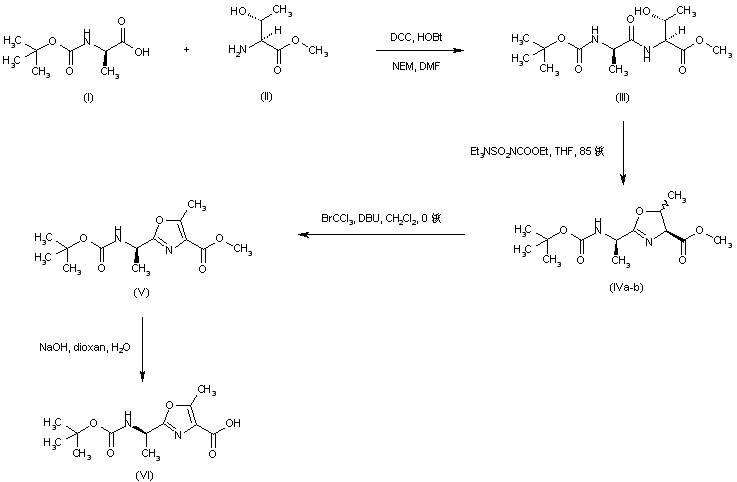 |
| 合成路线图解说明: The heterocyclic amino acid precursor (VI) was synthesized as follows. Coupling between N-Boc-D-alanine (I) and L-threonine methyl ester (II) in the presence of DCC and HOBt gave dipeptide (III). Cyclization of (III) using Burgess reagent provided the oxazoline (IV) as a mixture of diastereoisomers. Subsequent dehydrogenation of (IV) by means of bromotrichloromethane and DBU afforded the protected oxazole amino acid (V). Basic hydrolysis of the ethyl ester group then yielded the intermediate acid (VI). |
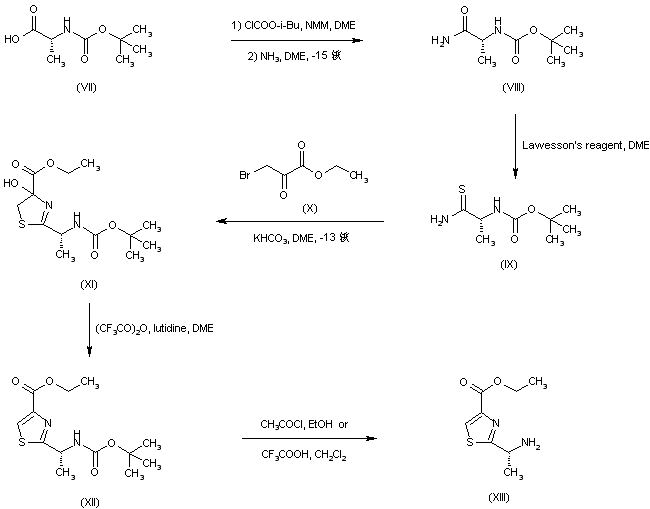 |
| 合成路线图解说明: N-Boc-D-alanine (VII) was converted into amide (VIII) via activation as the corresponding mixed anhydride with isobutyl chloroformate. Treatment of amide (VIII) with Lawesson抯 reagent gave thioamide (IX). Coupling of (IX) with ethyl bromopyruvate (X) produced the intermediate hydroxythiazoline (XI), which was further dehydrated using trifluoroacetic anhydride to afford the fully protected thiazole amino acid (XII). Removal of the Boc protecting group of (XII) to give (XIII) was achieved by treatment with either trifluoroacetic acid or acetyl chloride in EtOH. |
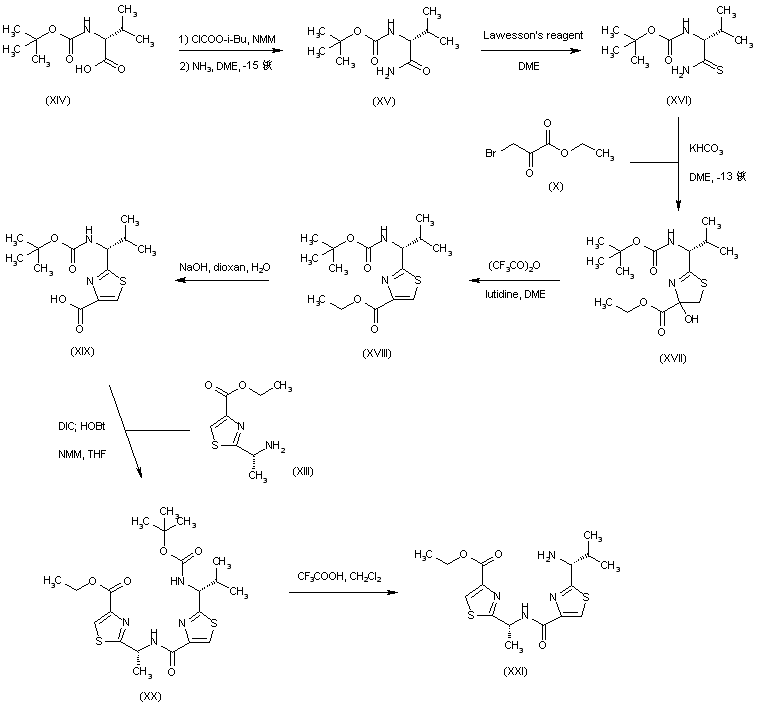 |
| 合成路线图解说明: The thiazole amino acid (XIX) was prepared by a similar method as above, consisting of conversion of N-Boc-D-valine (XIV) to the corresponding amide (XV) followed by thionation to (XVI). Condensation of thioamide (XVI) with ethyl bromopyruvate (X) and subsequent acid dehydration gave thiazole (XVIII). Basic hydrolysis of the ethyl ester group of (XVIII) then gave acid (XIX). Coupling of the N-Boc-amino acid (XIX) with aminoester (XIII) produced the corresponding amide (XX). Further acid cleavage of the Boc protecting group gave (XXI). |
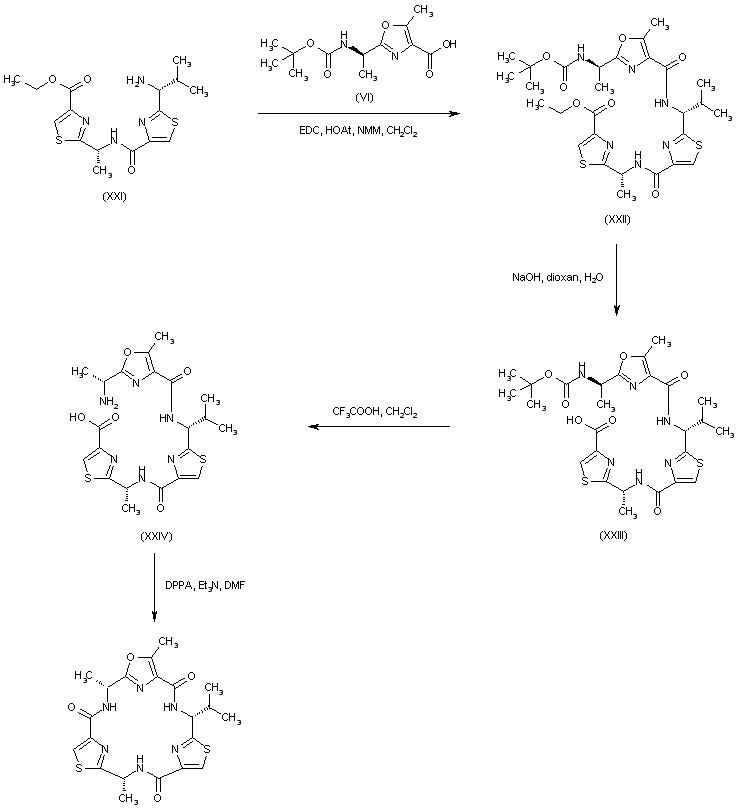 |
| 合成路线图解说明: The intermediate acid (VI) was then coupled to amine (XXI) to furnish (XXII). Sequential deprotection of the ethyl ester and the N-Boc protecting groups of (XXII) produced the linear precursor (XXIV). This was finally cyclized to the title compound using DPPA. |