| 参考文献No. | 574796 |
| 标题: | Syntheses of (R)- an (S)-2- and 6-fluoronorepinephrine and (R)- and (S)-2- and 6-fluoroepinephrine: Effect of stereochemistry on fluorine-induced adrenergic selectivities |
| 作者: | Lu, S.; Herbert, B.; Haufe, G.; Wilhelm Laue, K.; Padgett, W.L.; Oskunleti, O.; Daly, J.W.; Kirk, K.L. |
| 来源: | J Med Chem 2000,43(8),1611 |
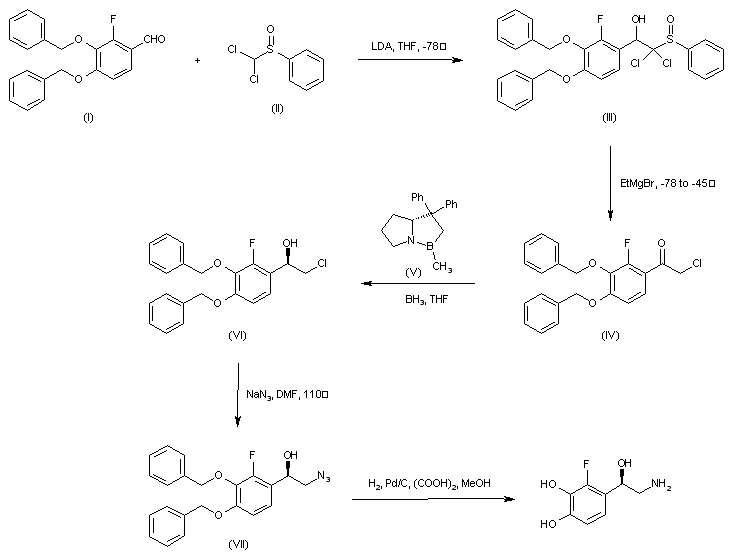 |
| 合成路线图解说明: The condensation of 3,4-dibenzyloxy-2-fluorobenzaldehyde (I) with dichloromethyl phenylsulfoxide (II) in the presence of LDA at -78 C afforded adduct (III) which, upon treatment with ethylmagnesium bromide gave rise to chloroketone (IV). Asymmetric carbonyl reduction of (IV) employing borane and the chiral oxazaborolidine catalyst (V) provided the (R)-chloroalcohol (VI). After conversion of (VI) to azide (VII), catalytic hydrogenation in the presence of Pd/C and oxalic acid yielded the title compound. |
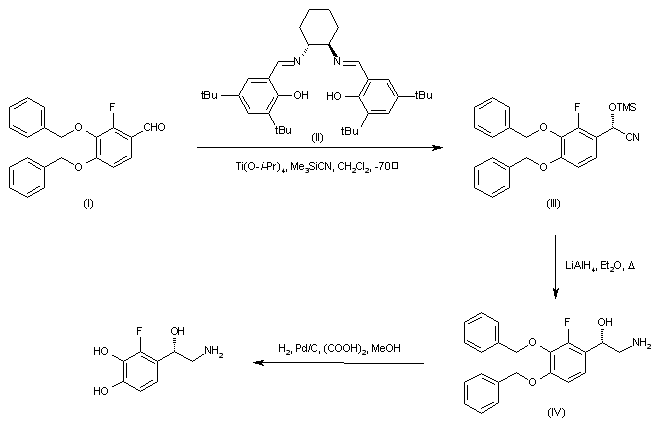 |
| 合成路线图解说明: Asymmetric addition of trimethylsilyl cyanide to 3,4-dibenzyloxy-2-fluorobenzaldehyde (I) employing the chiral (R,R)-salen ligand (II) in the presence of titanium tetraisopropoxide generated the chiral O-trimethylsilyl cyanohydrin (III), which was subsequently reduced to the desired (S)-aminoalcohol (IV) by means of LiAlH4. The benzyl protecting groups of (IV) were then removed by hydrogenolysis to furnish the title compound. |
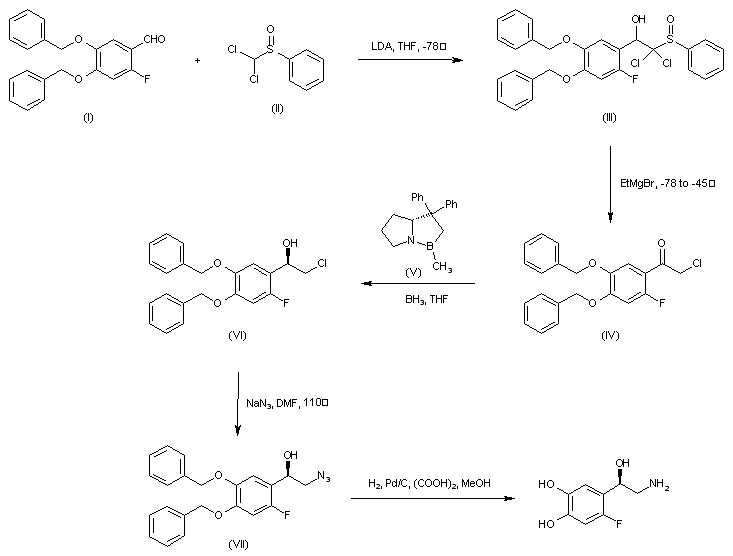 |
| 合成路线图解说明: The condensation of 4,5-dibenzyloxy-2-fluorobenzaldehyde (I) with dichloromethyl phenylsulfoxide (II) in the presence of LDA at -78 C afforded adduct (III) which, upon treatment with ethylmagnesium bromide gave rise to chloroketone (IV). Asymmetric carbonyl reduction of (IV) employing borane and the chiral oxazaborolidine catalyst (V) provided the (R)-chloroalcohol (VI). After conversion of (VI) to azide (VII), catalytic hydrogenation in the presence of Pd/C and oxalic acid yielded the title compound. |
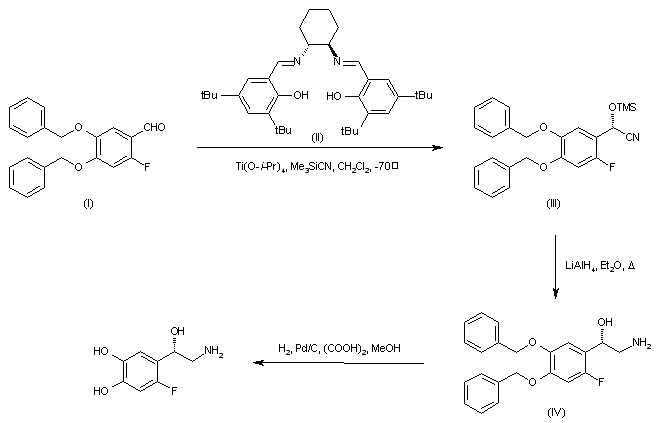 |
| 合成路线图解说明: Asymmetric addition of trimethylsilyl cyanide to 4,5-dibenzyloxy-2-fluorobenzaldehyde (I) employing the chiral (R,R)-salen ligand (II) in the presence of titanium tetraisopropoxide generated the chiral O-trimethylsilyl cyanohydrin (III), which was subsequently reduced to the desired (S)-aminoalcohol (IV) by means of LiAlH4. The benzyl protecting groups of (IV) were then removed by hydrogenolysis to furnish the title compound. |
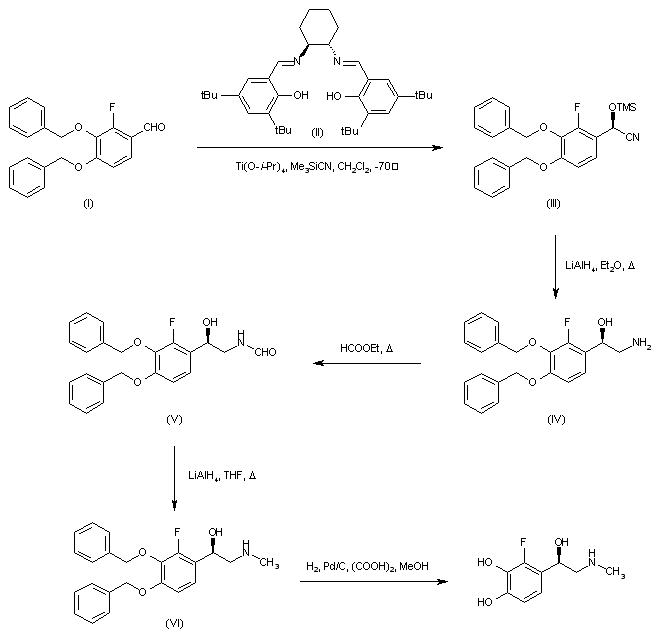 |
| 合成路线图解说明: Asymmetric addition of trimethylsilyl cyanide to 3,4-dibenzyloxy-2-fluorobenzaldehyde (I) employing the chiral (S,S)-salen ligand (II) in the presence of titanium tetraisopropoxide generated the chiral O-trimethylsilyl cyanohydrin (III), which was subsequently reduced to the desired (R)-aminoalcohol (IV) by means of LiAlH4. Heating of (IV) with ethyl formate provided formamide (V), which was reduced to the N-methyl amine (VI) with LiAlH4 in refluxing THF. The benzyl protecting groups of (VI) were finally removed by hydrogenolysis to furnish the title compound. |
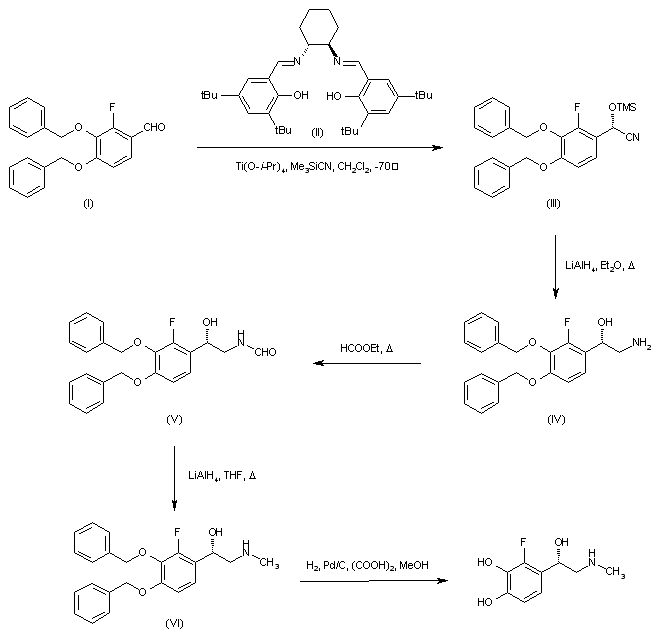 |
| 合成路线图解说明: Asymmetric addition of trimethylsilyl cyanide to 3,4-dibenzyloxy-2-fluorobenzaldehyde (I) employing the chiral (R,R)-salen ligand (II) in the presence of titanium tetraisopropoxide generated the chiral O-trimethylsilyl cyanohydrin (III), which was subsequently reduced to the desired (S)-aminoalcohol (IV) by means of LiAlH4. Heating of (IV) with ethyl formate provided formamide (V), which was reduced to the N-methyl amine (VI) with LiAlH4 in refluxing THF. The benzyl protecting groups of (VI) were finally removed by hydrogenolysis to furnish the title compound. |
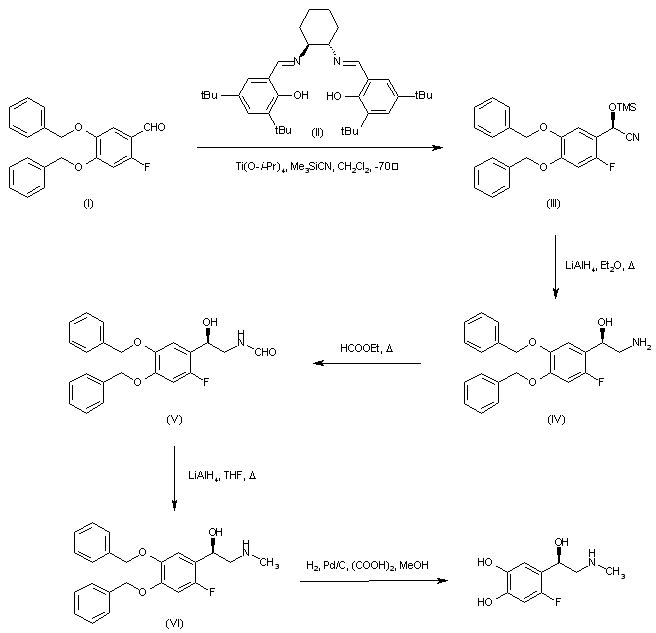 |
| 合成路线图解说明: Asymmetric addition of trimethylsilyl cyanide to 4,5-dibenzyloxy-2-fluorobenzaldehyde (I) employing the chiral (S,S)-salen ligand (II) in the presence of titanium tetraisopropoxide generated the chiral O-trimethylsilyl cyanohydrin (III), which was subsequently reduced to the desired (R)-aminoalcohol (IV) by means of LiAlH4. Heating of (IV) with ethyl formate provided formamide (V), which was reduced to the N-methyl amine (VI) with LiAlH4 in refluxing THF. The benzyl protecting groups of (VI) were finally removed by hydrogenolysis to furnish the title compound. |
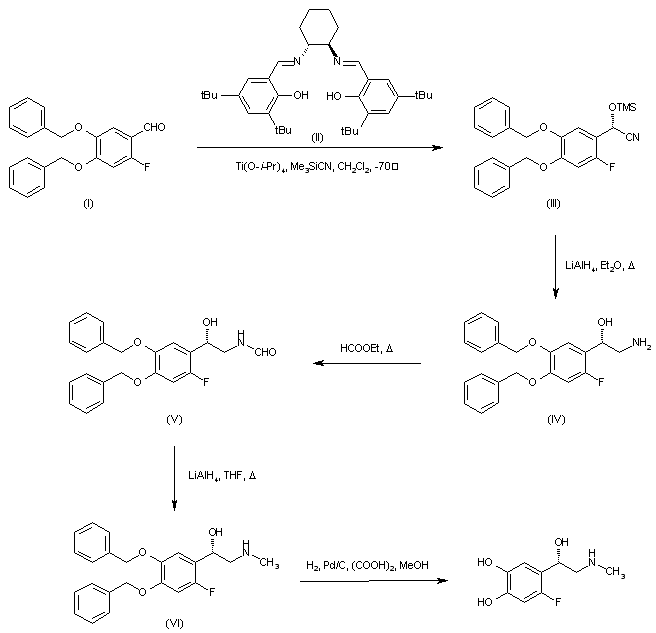 |
| 合成路线图解说明: Asymmetric addition of trimethylsilyl cyanide to 4,5-dibenzyloxy-2-fluorobenzaldehyde (I) employing the chiral (R,R)-salen ligand (II) in the presence of titanium tetraisopropoxide generated the chiral O-trimethylsilyl cyanohydrin (III), which was subsequently reduced to the desired (S)-aminoalcohol (IV) by means of LiAlH4. Heating of (IV) with ethyl formate provided formamide (V), which was reduced to the N-methyl amine (VI) with LiAlH4 in refluxing THF. The benzyl protecting groups of (VI) were finally removed by hydrogenolysis to furnish the title compound. |