| 参考文献No. | 23885 |
| 标题: | Tricyclic cpds. with pharmaceutical activity |
| 作者: | Boyle, F.; Crook, J.W.; Matusiak, Z.S. (AstraZeneca plc; BTG plc) |
| 来源: | EP 0667864; GB 2272217; JP 1996505838; US 5789417; WO 9411354 |
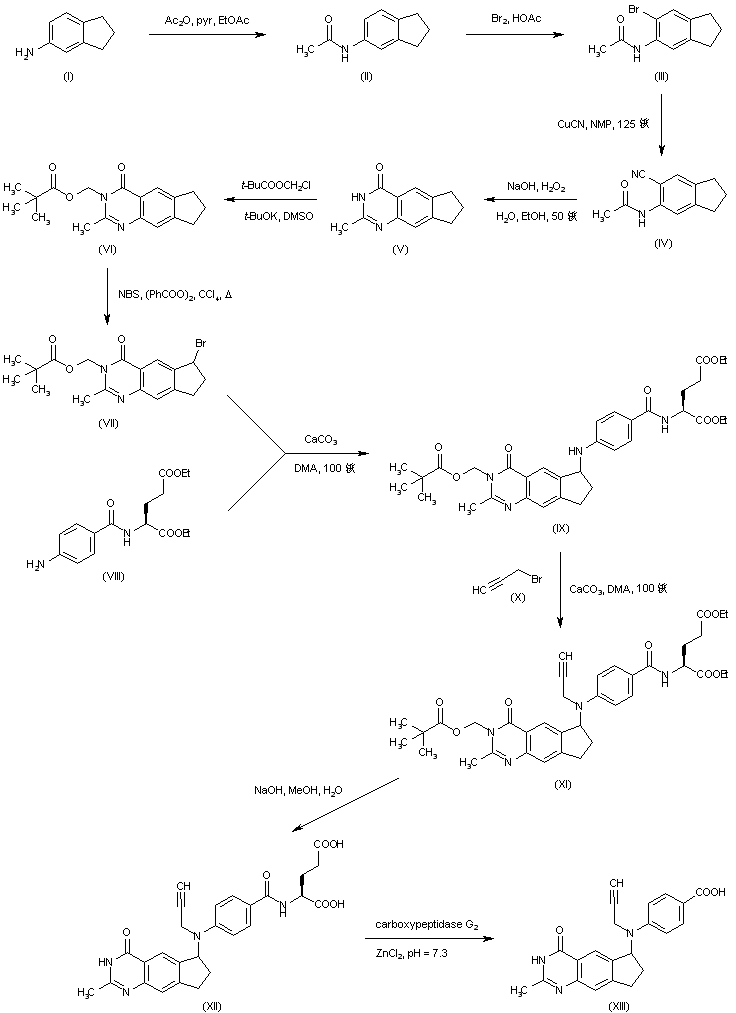 |
| 合成路线图解说明: 5-Aminoindan (I) was acetylated, and the resulting anilide (II) was brominated in HOAc to afford (III). Displacement of the bromine of (III) with CuCN in N-methylpyrrolidinone at 125 C furnished nitrile (IV), which was cyclized to the desired quinazolinone derivative (V) upon treatment with sodium hydroperoxide. Protection of the NH group of (V) was achieved by alkylation with chloromethyl pivaloate yielding (VI). Benzylic bromination of (VI) with N-bromosuccinimide in the presence of benzoyl peroxide gave bromide (VII). This was condensed with diethyl p-aminobenzoyl-L-glutamate (VIII) by means of CaCO3 to produce adduct (IX). Further alkylation of (IX) with propargyl bromide (X) using CaCO3 provided propargyl amine (XI). Hydrolysis of the ethyl esters and pivaloyloxymethyl protecting group of (XI) with NaOH afforded (XII). The glutamic acid moiety of (XII) was then removed by enzimatic hydrolysis with carboxypeptidase G2 to furnish intermediate (XIII). |
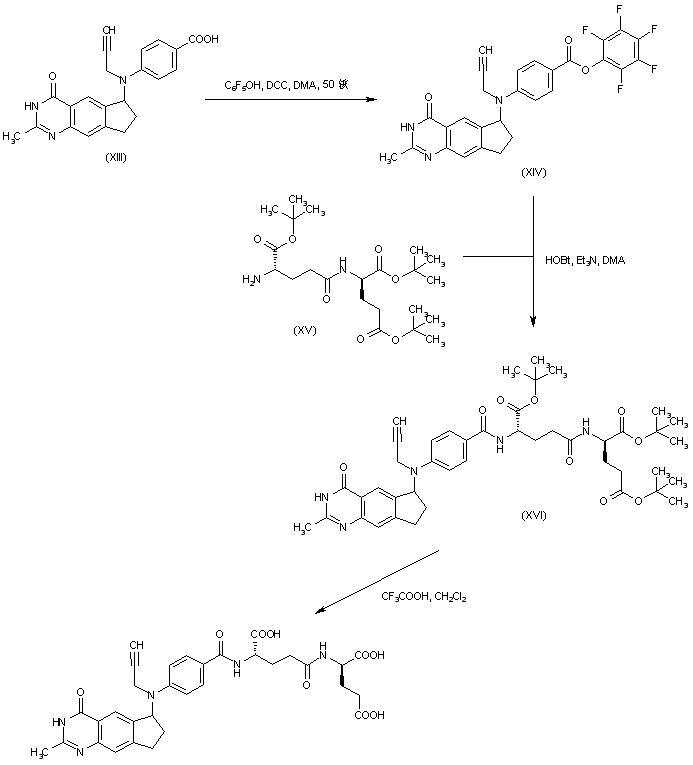 |
| 合成路线图解说明: Benzoic acid derivative (XIII) was converted to pentafluorophenyl ester (XIV) by DCC-mediated coupling with pentafluorophenol. Then, condensation with tri-tert-butyl L-gamma-glutamyl-D-glutamate (XV) furnished the corresponding amide (XVI). The tert-butyl esters of (XVI) were finally cleaved by treatment with trifluoroacetic acid. |