| 参考文献No. | 49810 |
| 标题: | Synthetic polysaccharides, their method of production and pharmaceutical compsns. containing same |
| 作者: | Petitou, M.; Herbert, J.-M.; Duchaussoy, P.; Basten, J.; Dreef-Tromp, C.; Driguez, P.-A.; Van Boeckel, C. (Akzo Nobel N.V.; Sanofi-Synth閘abo) |
| 来源: | EP 1049721; FR 2773804; US 6528497; WO 9936443 |
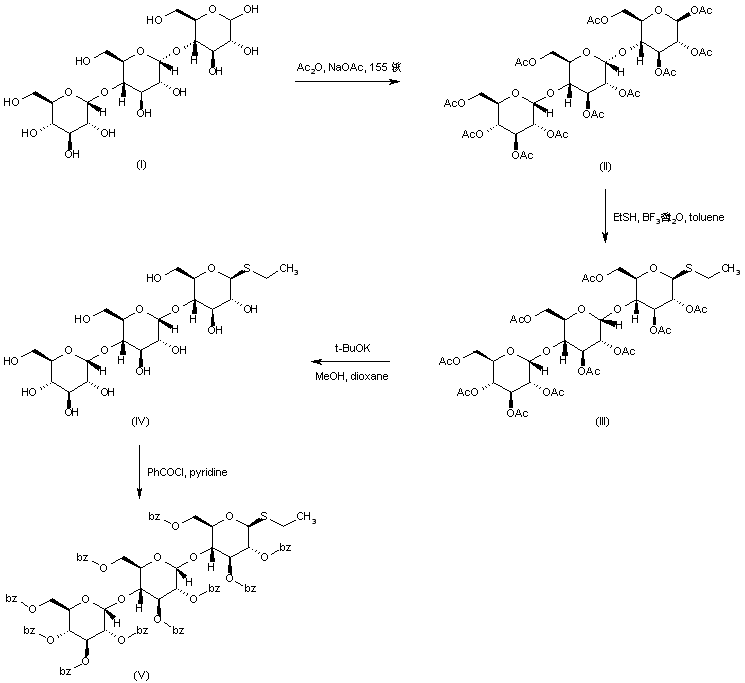 |
| 合成路线图解说明: The intermediate thioglycoside (V) is prepared as follows. Esterification of maltotriose (I) with acetic anhydride and sodium acetate provides peracetate (II). Subsequent reaction of (II) with ethanethiol in the presence of boron trifluoride etherate yields the ethyl thiopyranoside (III). Removal of the acetate esters of (III) is effected by methanolysis in the presence of potassium tert-butoxide, yielding (IV). Then, reesterification of (IV) by means of benzoyl chloride and pyridine furnishes the target intermediate (V). |
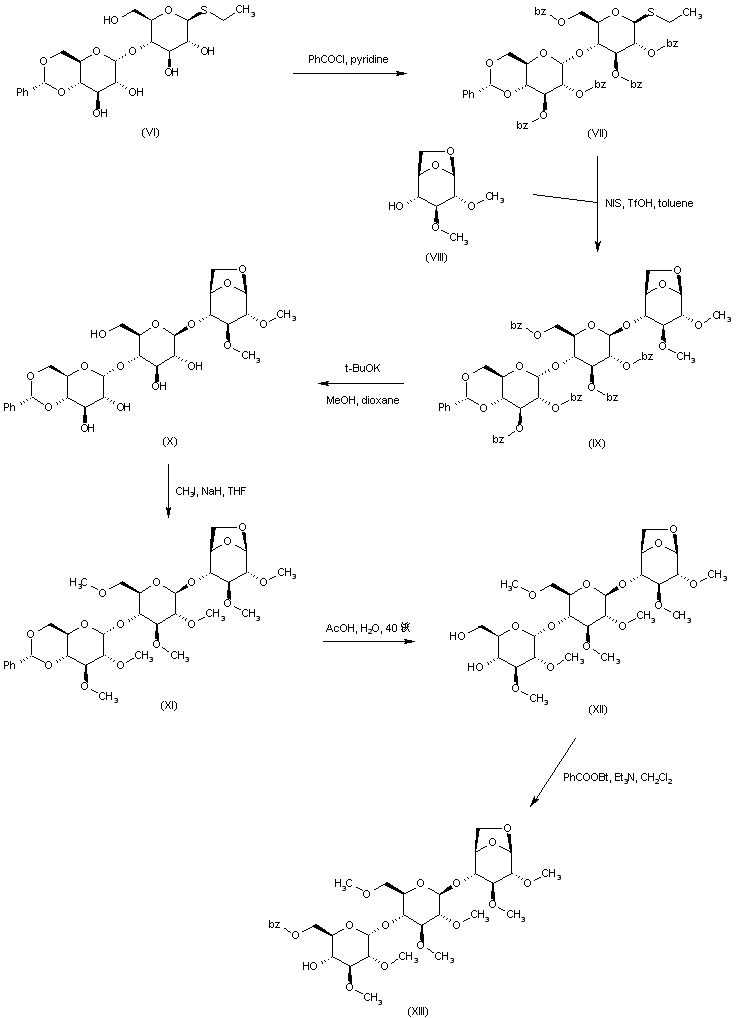 |
| 合成路线图解说明: The known disaccharide thioglucoside (VI) is esterified by means of benzoyl chloride and pyridine to give the penta-benzoate (VII). Coupling between thioglycoside (VII) and 2,3-di-O-methyl-1,6-anhydro-D-glucose (VIII) is effected by treatment with N-iodosuccinimide and trifluoromethanesulfonic acid, yielding trisaccharide (IX). After methanolysis of the benzoate esters, the resultant penta-hydroxy compound (X) is alkylated with iodomethane and NaH, leading to the corresponding methyl ether (XI). Hydrolysis of the benzylidene acetal of (XI) with 80% AcOH provides diol (XII). The primary hydroxyl group of (XII) is then selectively esterified by using 1-(benzoyloxy)-benzotriazole to produce the mono-benzoate (XIII). |
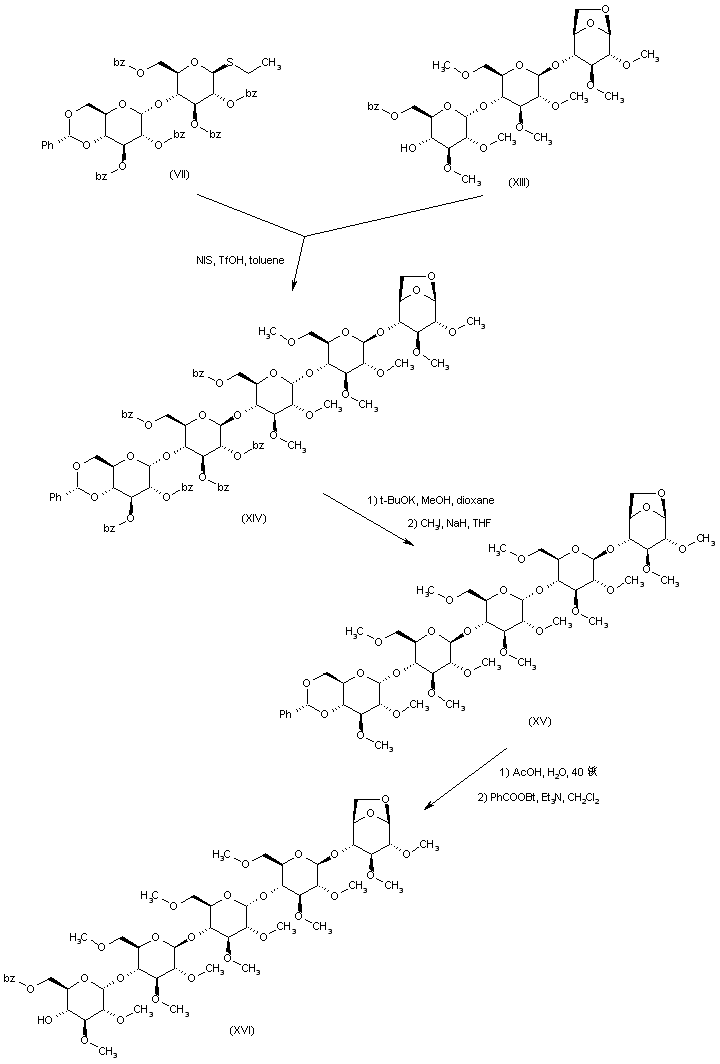 |
| 合成路线图解说明: Coupling between trisaccharide (XIII) and thioglycoside (VII) using N-iodosuccinimide/trifluoromethanesulfonic acid furnishes pentasaccharide (XIV). After methanolysis of the benzoate esters of (XIV), the poly-methyl ether (XV) is prepared by methylation of (XIV) with iodomethane and NaH. Acidic hydrolysis of the benzylidene acetal (XV), followed by selective esterification with 1-(benzoyloxy)-benzotriazole affords benzoate (XVI). |
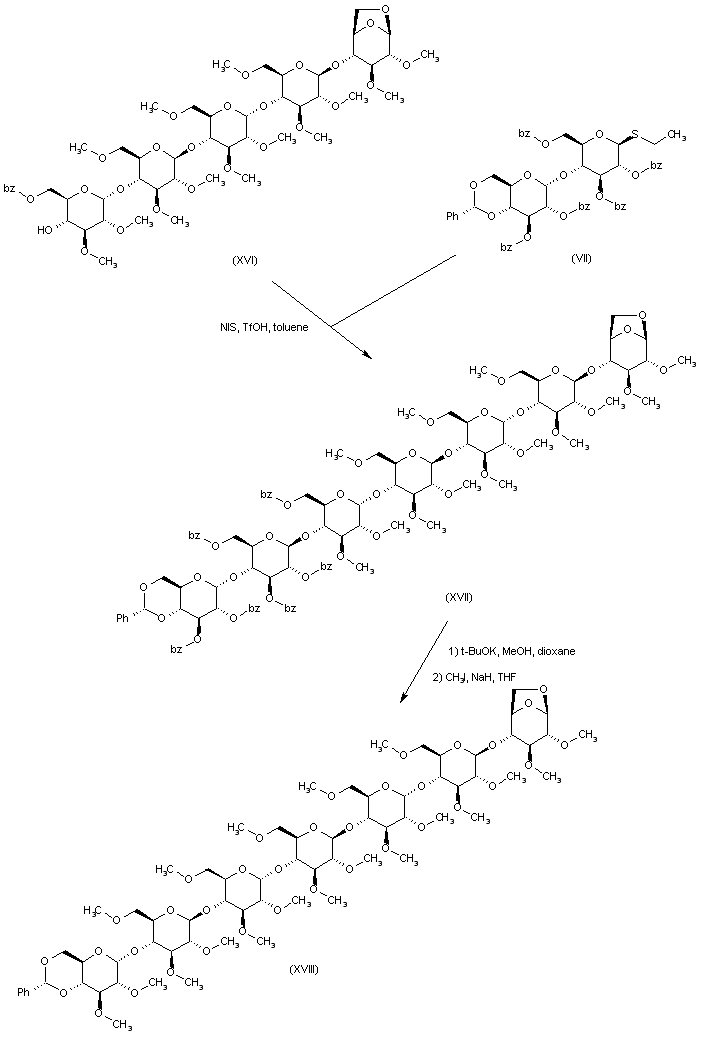 |
| 合成路线图解说明: A new coupling of pentasaccharide (XVI) with thioglycoside (VII) produces heptasaccharide (XVII). Hydrolysis of the benzoate esters of (XVII), followed by O-methylation, yields methyl ether (XVIII). |
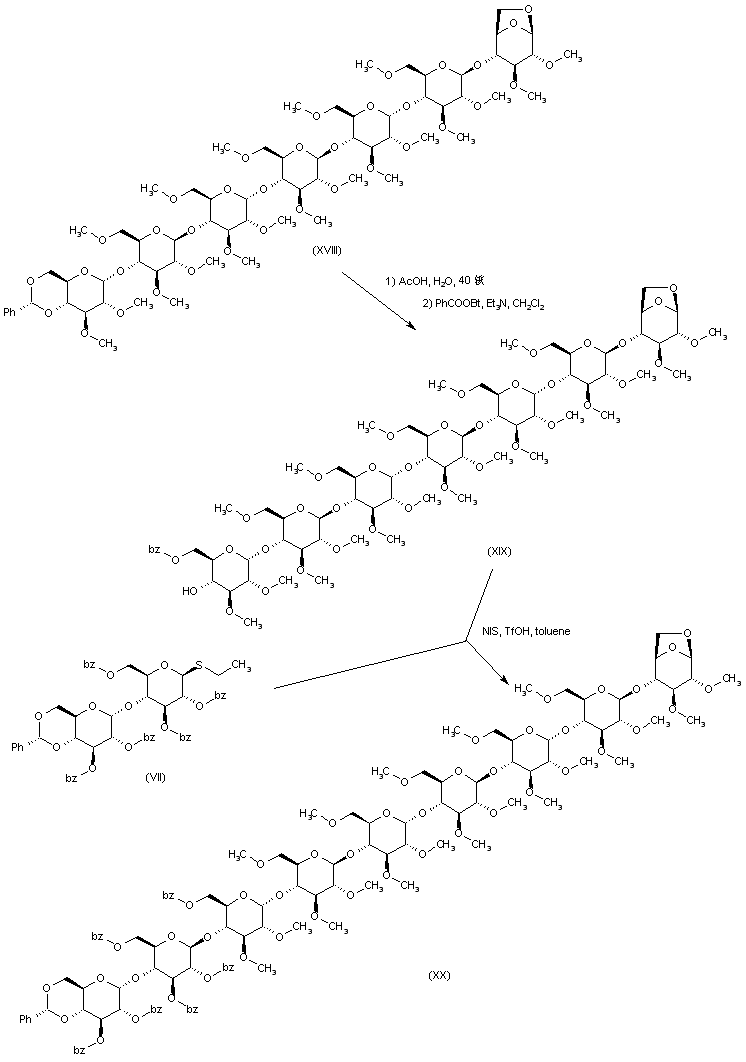 |
| 合成路线图解说明: After acidic hydrolysis of the benzylidene acetal of (XVIII), esterification with 1-(benzoyloxy)-benzotriazole leads to (XIX). This is then coupled with a third moiety of disaccharide thioglycoside (VII), producing (XX). |
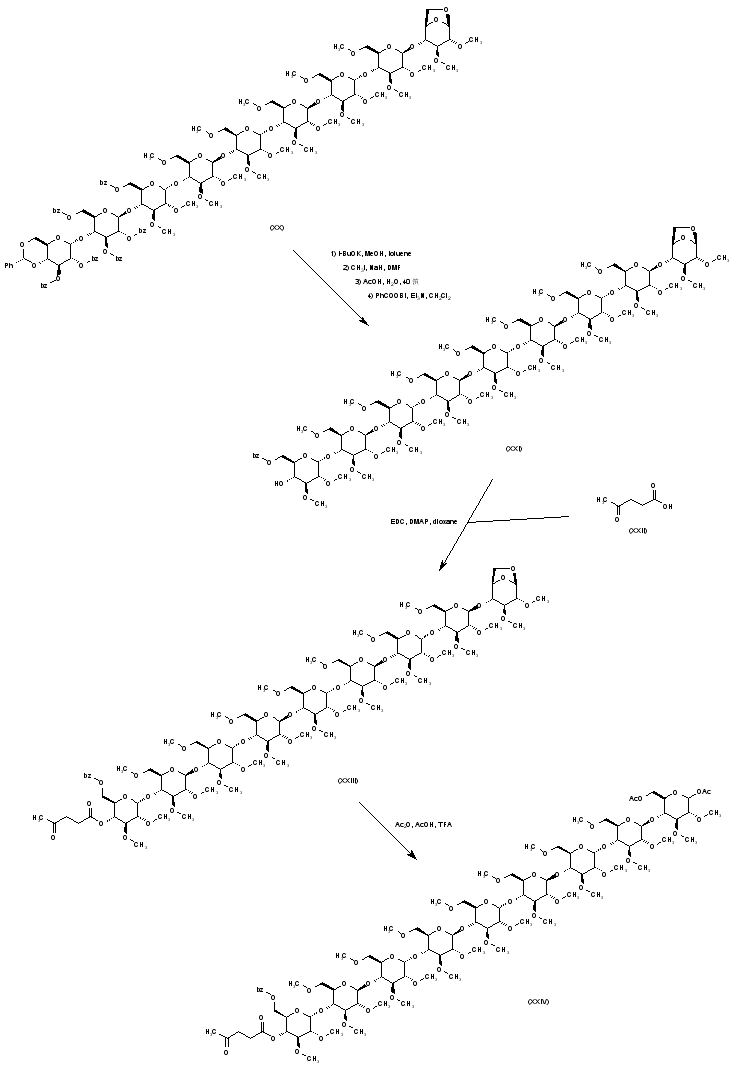 |
| 合成路线图解说明: The nonasaccharide (XX) is transformed into (XXI) by the sequence of benzoate esters hydrolysis, O-methylation, acetal hydrolysis, and selective mono-benzoylation, following the same methods as above. The free hydroxyl group of (XXI) is subsequently protected by esterification with levulinic acid (XXII) to give levulinate ester (XXIII). Opening of the 1,6-anhydroglucose unit of (XXIII) is then accomplished by treatment with acetic anhydride in the presence of acetic acid and trifluoroacetic acid, producing (XXIV). |
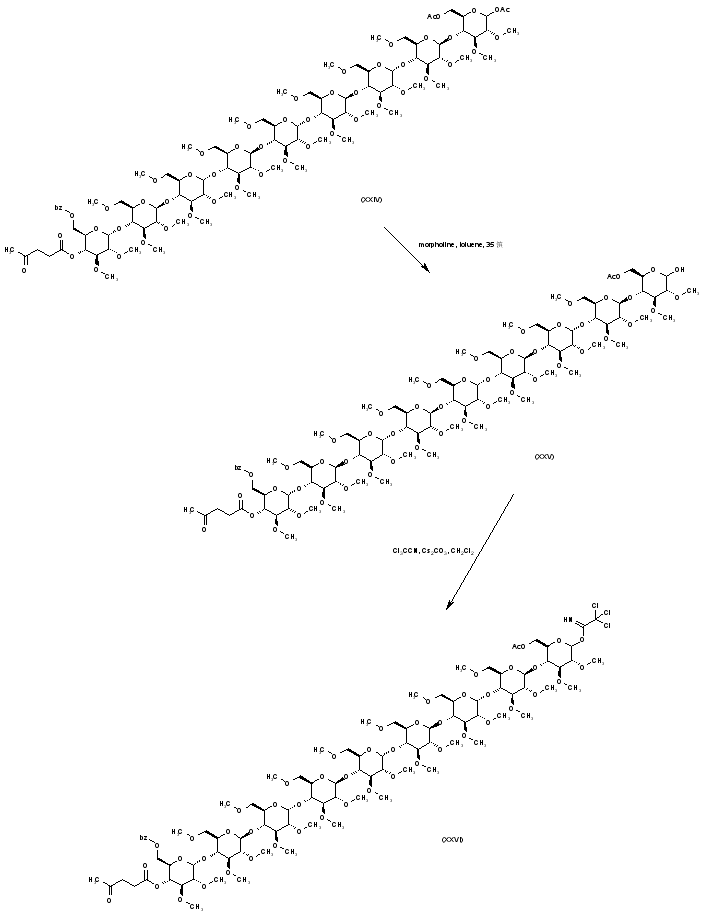 |
| 合成路线图解说明: The glycosidic acetate group of (XXIV) is selectively hydrolyzed to (XXV) by means of morpholine in toluene. Activation of (XXV) by means of trichloroacetonitrile produces imidate (XXVI). |
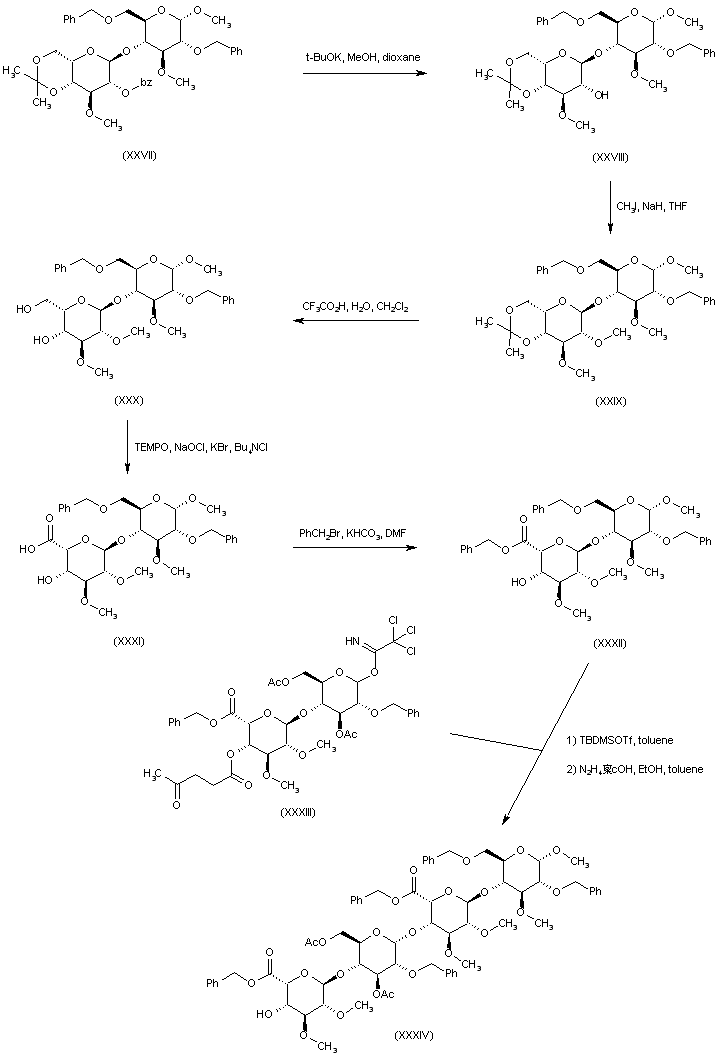 |
| 合成路线图解说明: Preparation of the tetrasaccharide building block (XXXIV) is carried out as follows. The disaccharide benzoate ester (XXVII) is hydrolyzed to alcohol (XXVIII), which is subsequently alkylated with iodomethane and NaH to the methyl ether (XXIX). Acidic hydrolysis of the acetonide group of (XXIX) provides diol (XXX). The primary alcohol of (XXX) is then oxidized by means of TEMPO and NaOCl, yielding carboxylic acid (XXXI), which is further converted to the benzyl ester (XXXII) upon treatment with benzyl bromide and KHCO3. Coupling of the disaccharide acceptor (XXXII) with imidate (XXXIII) in the presence of tert-butyldimethylsilyl triflate, followed by removal of the levulinate ester with hydrazine acetate, furnishes tetrasaccharide (XXXIV). |
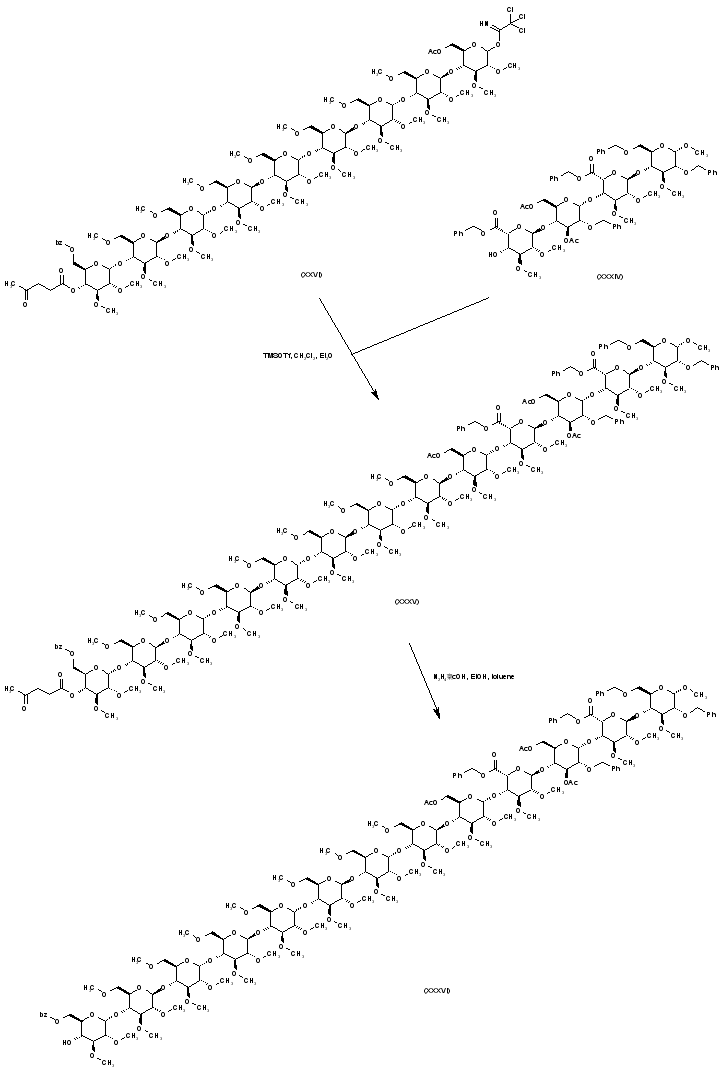 |
| 合成路线图解说明: The nonasaccharide imidate (XXVI) is coupled to the tetrasaccharide acceptor (XXXIV) to provide adduct (XXXV). Subsequent deprotection of the levulinate ester of (XXXV) to afford (XXXVI) is effected by treatment with hydrazine acetate. |
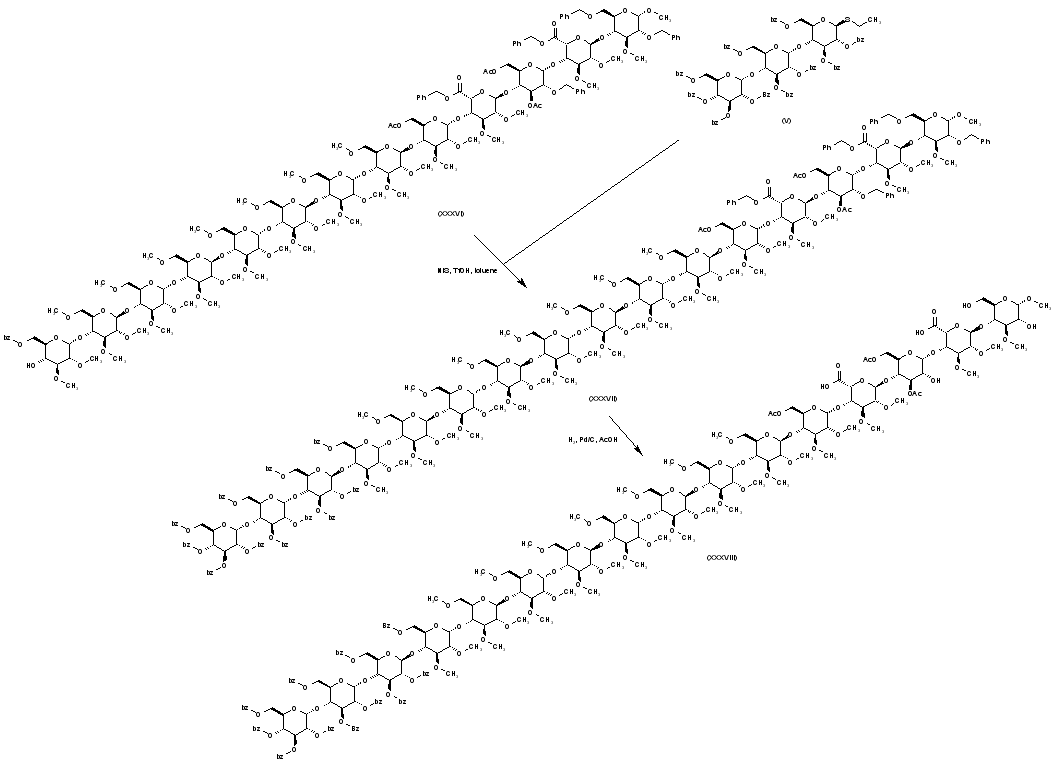 |
| 合成路线图解说明: Coupling of polysaccharide (XXXVI) with the thioglycoside (V) using N-iodosuccinimide and trifluromethanesulfonic acid provides (XXXVII). The benzyl ether and ester groups of (XXXVII) are then removed by hydrogenolysis in the presence of Pd/C to produce (XXXVIII). |
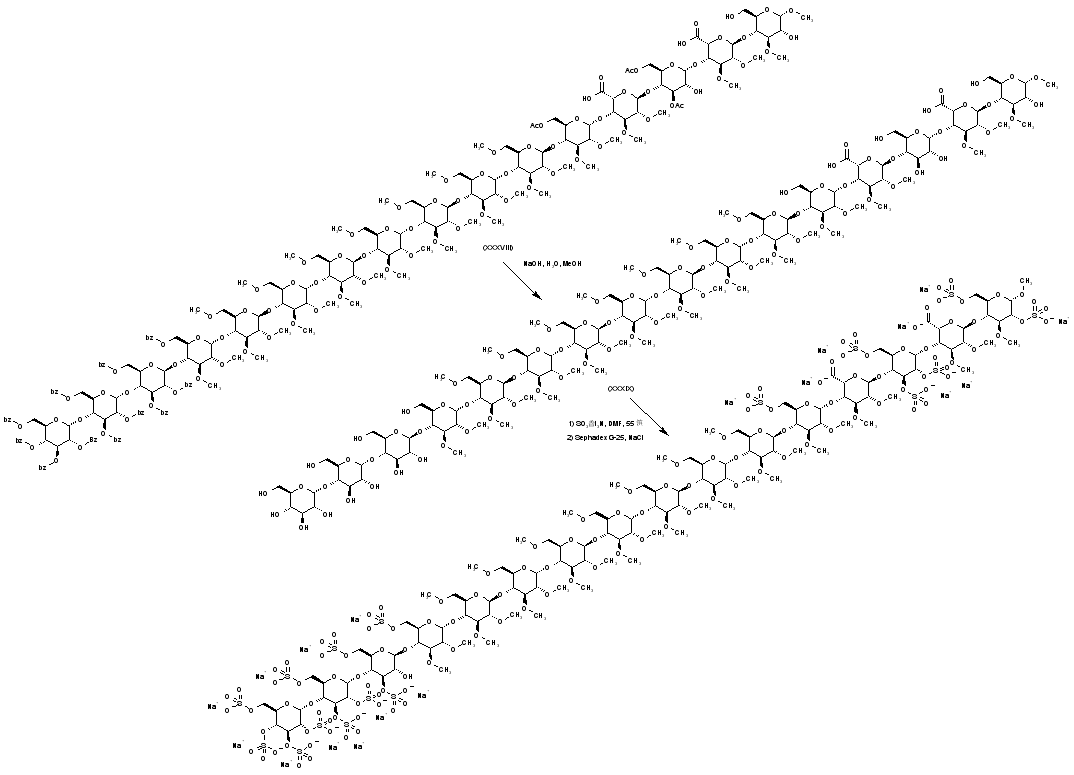 |
| 合成路线图解说明: Basic hydrolysis of the acetate and benzoate ester groups of (XXXVIII) produces the polyol (XXXIX). Then, sulfation of the free hydroxyl groups of (XXXIX) by means of sulfur trioxide-triethylamine complex leads to the target polysulfate derivative, which is finally isolated as the corresponding sodium salt by chromatography on Sephadex? eluting with NaCl. |