| 参考文献No. | 54280 |
| 标题: | Selected fused pyrrolocarbazoles |
| 作者: | Hudkins, R.L.; Gingrich, D.E. (Cephalon, Inc.) |
| 来源: | WO 0217914 |
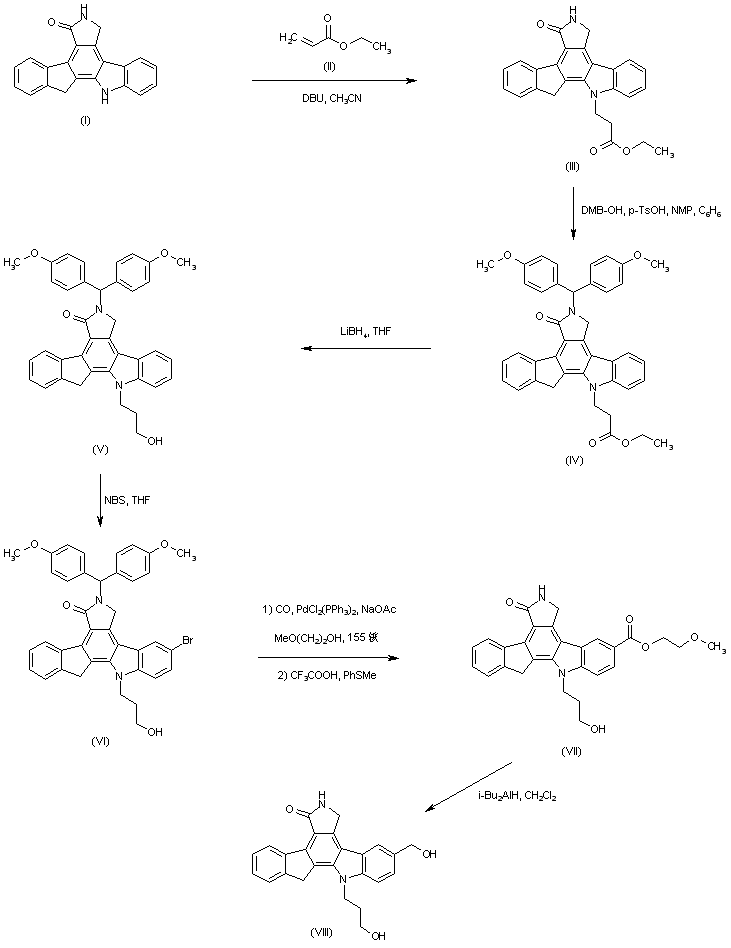 |
| 合成路线图解说明: Michael addition of ethyl acrylate (II) to the indenopyrrolocarbazole (I) provided (III). Lactam N-protection of (III) with dimethoxybenzhydrol yielded N-protected ethyl ester (IV), which was reduced with LiBH4 to give alcohol (V). Subsequent bromination of (V) with N-bromosuccinimide yielded (VI), which was first submitted to palladium-catalyzed carbonylation in methoxyethanol and then N-deprotected with trifluoroacetic acid and thioanisole to furnish (VII). Finally, reduction of ester (VII) with DIBAL furnished the diol intermediate (VIII). |
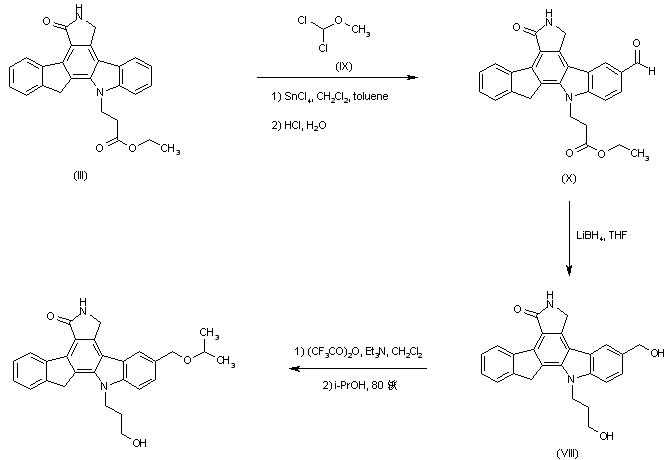 |
| 合成路线图解说明: An alternative route was based on the formylation of (III) with dichloromethyl methyl ether (IX) in the presence of tin chloride. The resultant aldehyde ester (X) was then reduced to diol (VIII) by means of LiBH4. The crude trifluoroacetate ester obtained by treatment of diol (VIII) with trifluoroacetic anhydride was then refluxed with isopropanol to furnish the title isopropyl ether. |
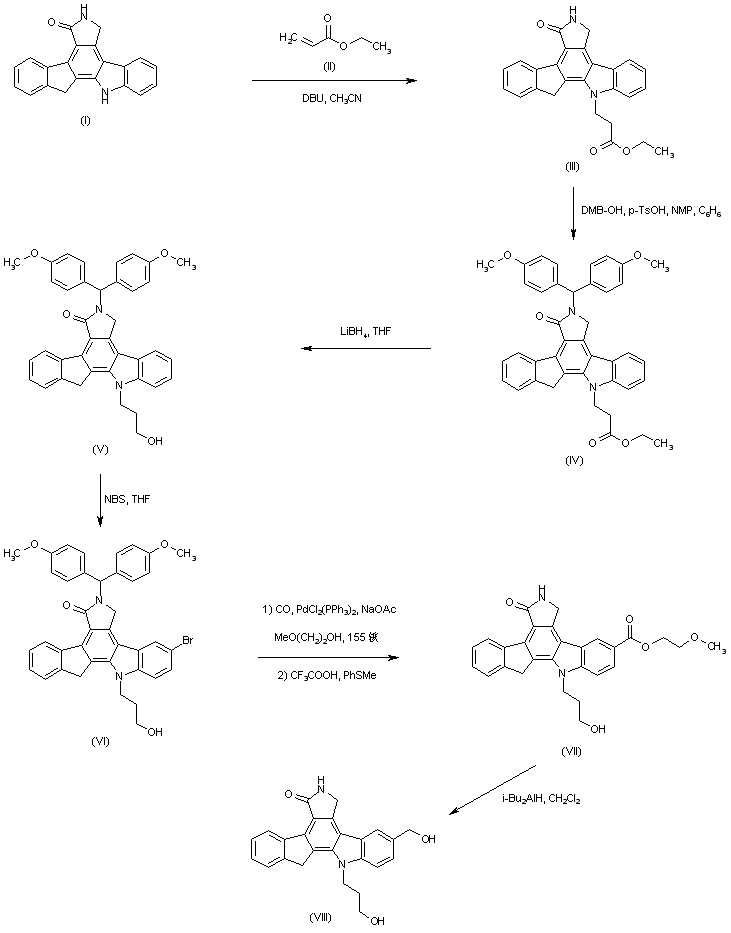 |
| 合成路线图解说明: Michael addition of ethyl acrylate (II) to the indenopyrrolocarbazole (I) provided (III). Lactam N-protection of (III) with dimethoxybenzhydrol yielded N-protected ethyl ester (IV), which was reduced with LiBH4 to give alcohol (V). Subsequent bromination of (V) with N-bromosuccinimide yielded (VI), which was first submitted to palladium-catalyzed carbonylation in methoxyethanol and then N-deprotected with trifluoroacetic acid and thioanisole to furnish (VII). Finally, reduction of ester (VII) with DIBAL furnished the diol intermediate (VIII). |
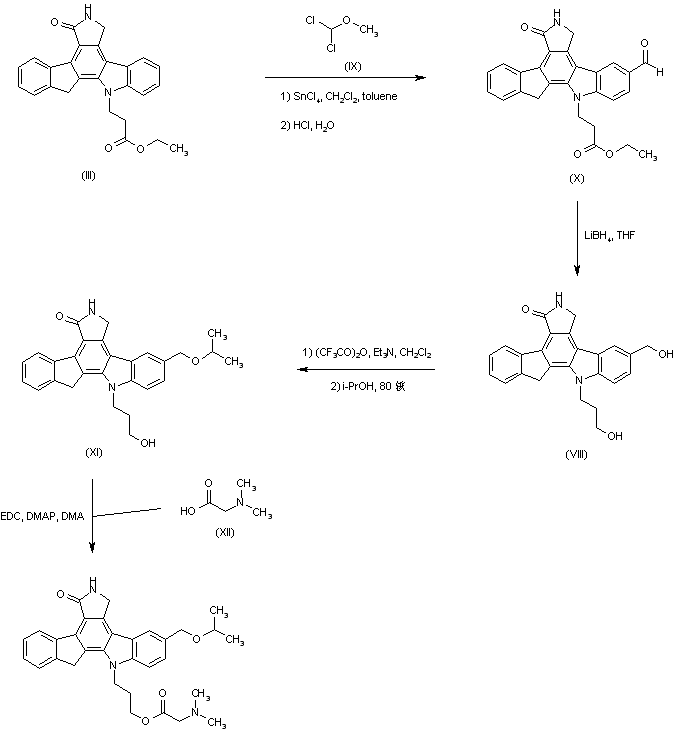 |
| 合成路线图解说明: An alternative route was based on the formylation of (III) with dichloromethyl methyl ether (IX) in the presence of tin chloride. The resultant aldehyde ester (X) was then reduced to the already reported diol (VIII) by means of LiBH4. The crude trifluoroacetate ester obtained by treatment of diol (VIII) with trifluoroacetic anhydride was then refluxed with isopropanol to furnish the isopropyl ether (XI). The remaining hydroxyl group was finally esterified by treatment with N,N-dimethylglycine (XII) in the presence of EDC and DMAP. |