| 参考文献No. | 534134 |
| 标题: | Synthesis and biological evaluation of highly functionalized analogues of ingenol |
| 作者: | Winkler, J.D.; Lewin, N.E.; Blumberg, P.M.; Harrison, S.; Kim, S. |
| 来源: | J Am Chem Soc 1999,121(2),296 |
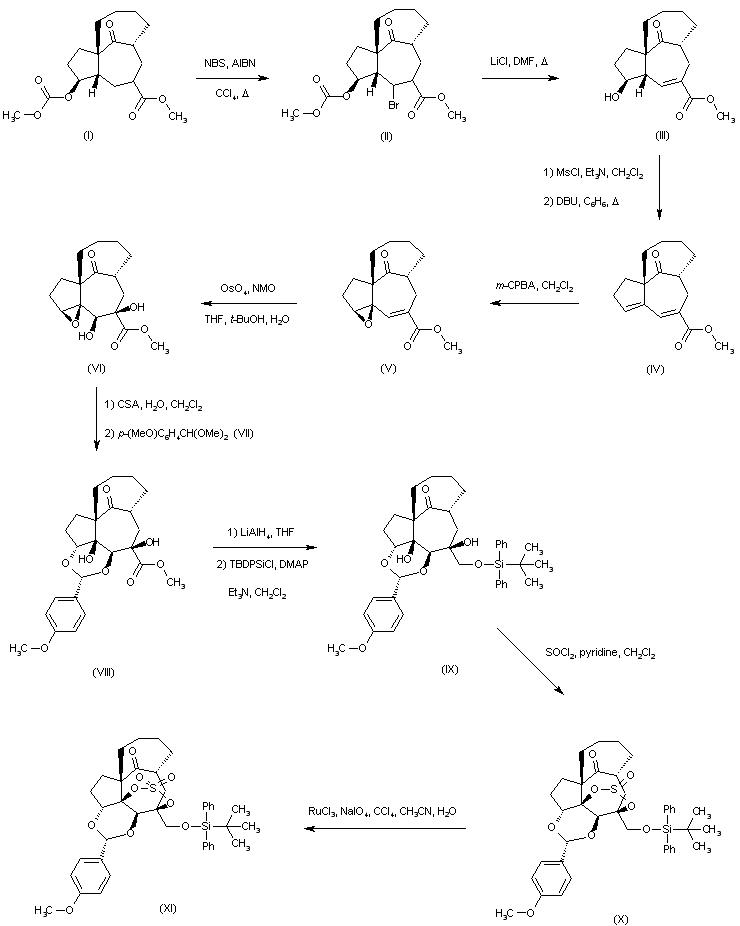 |
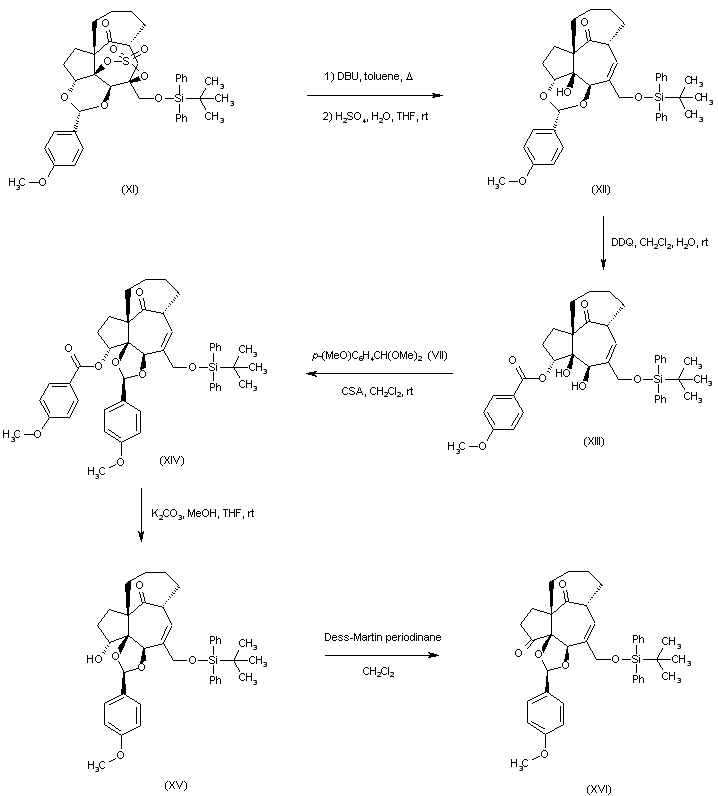
| 合成路线图解说明: The known photoaddition-fragmentation product (I) was brominated with N-bromosuccinimide in the presence of AIBN to afford bromoester (II), which upon treatment with LiCl in refluxing DMF produced the unsaturated ester (III) with concomitant removal of the methyl carbonate. Subsequent mesylation of the hydroxyl group of (III), followed by elimination with DBU in boiling benzene led to the formation of the diene ester (IV). Selective epoxidation of (IV) with m-chloroperbenzoic acid yielded the epoxy-unsaturated ester (V), which was oxidized with OsO4 and N-methylmorpholine-N-oxide to the epoxy diol (VI). Reaction of (VI) with camphorsulfonic acid in wet CH2Cl2 gave the corresponding tetraol, which on exposure to p-anisaldehyde dimethyl acetal (VII) produced selectively the acetal (VIII). The reduction of the ester group of (VIII) with LiAlH4, followed by silylation with TBDPSi-Cl afforded the monosilylated compound (IX). The reaction of (IX) with SOCl2 in the presence of pyridine generated the cyclic sulfite ester (X), which was oxidized to sulfate (XI) using RuCl3 and NaIO4. |
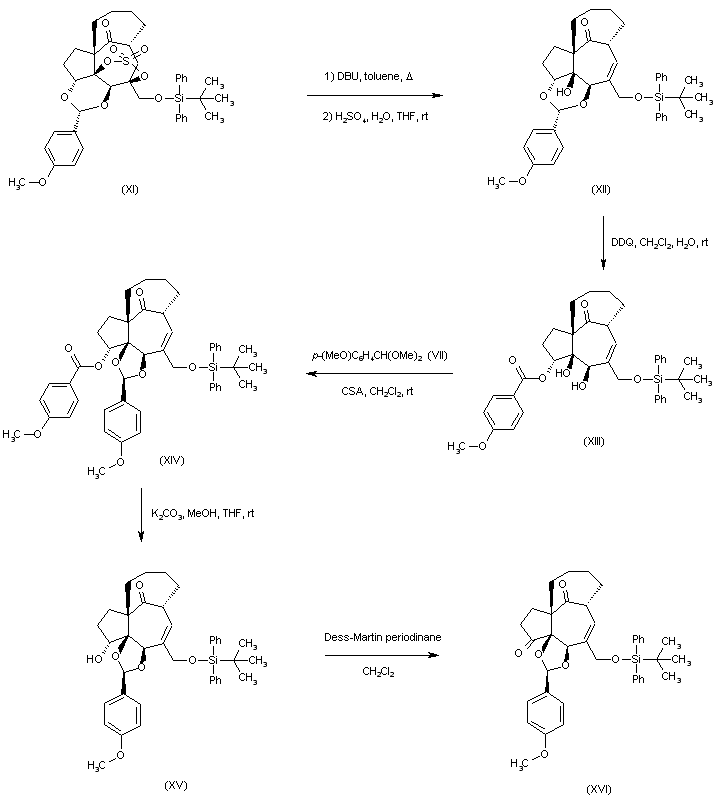 |
| 合成路线图解说明: Elimination of the sulfate group of (XI) with DBU in refluxing toluene led, after acidic workup, to the formation of the allylic alcohol (XII). Oxidative cleavage of the p-methoxyphenyl acetal of (XII) with DDQ produced regioselectively the p-methoxybenzoate ester (XIII). Further treatment of (XIII) with p--anisaldehyde dimethyl acetal (VII) gave the acetal (XIV). Ester hydrolysis of (XIV), followed by oxidation of the resulting alcohol (XV) with Dess-Martin periodinane afforded ketone (XVI). |
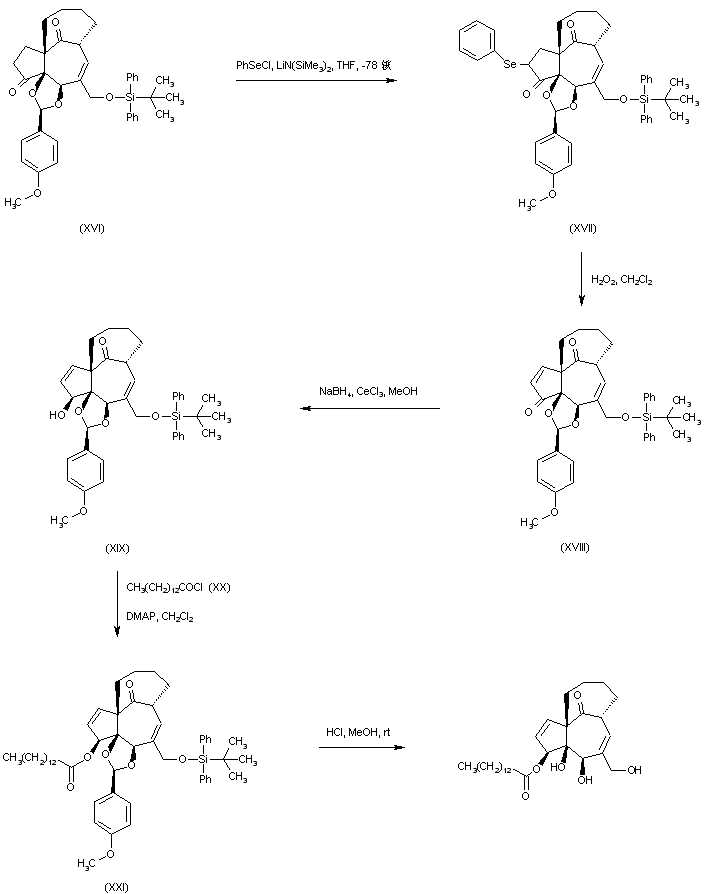 |
| 合成路线图解说明: Ketone (XVI) was treated with phenylselanyl chloride and LHMDS yielding the selenium derivative (XVII). Enone (XVIII) was then produced by oxidative elimination of the selenide (XVII). Reduction of the unsaturated ketone (XVIII) with NaBH4 in the presence of CeCl3 gave the 3-beta-allylic alcohol (XIX). This was acylated with myristoyl chloride (XX) to afford ester (XXI). Finally, acid deprotection of the silyl ether and the p-methoxyphenyl acetal of (XXI) furnished the title compound. |
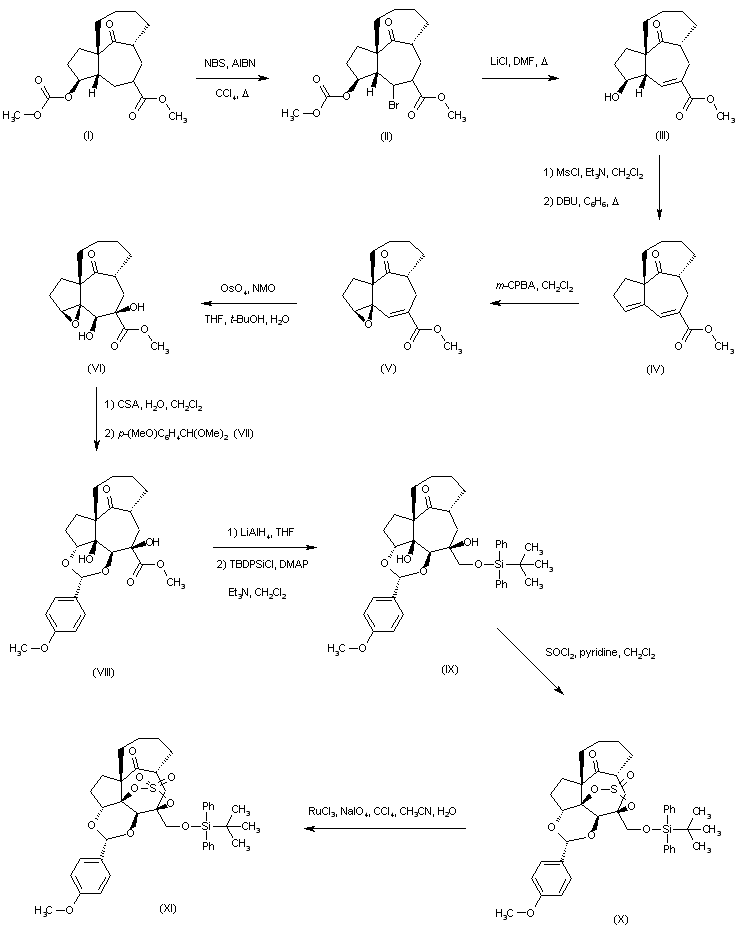 |
| 合成路线图解说明: The known photoaddition-fragmentation product (I) was brominated with N-bromosuccinimide in the presence of AIBN to afford bromoester (II), which upon treatment with LiCl in refluxing DMF produced the unsaturated ester (III) with concomitant removal of the methyl carbonate. Subsequent mesylation of the hydroxyl group of (III), followed by elimination with DBU in boiling benzene led to the formation of the diene ester (IV). Selective epoxidation with m-chloroperbenzoic acid yielded the epoxy-unsaturated ester (V), which was oxidized with OsO4 and N-methylmorpholine-N-oxide to the epoxy diol (VI). Reaction of (VI) with camphorsulfonic acid in wet CH2Cl2 gave the corresponding tetraol, which on exposure to p-anisaldehyde dimethyl acetal (VII) produced selectively the acetal (VIII). The reduction of the ester group of (VIII) with LiAlH4, followed by silylation with TBDPSi-Cl afforded the monosilylated derivative (IX). The reaction of (IX) with SOCl2 in the presence of pyridine generated the cyclic sulfite ester (X), which was oxidized to sulfate (XI) using RuCl3 and NaIO4. |
 |
| 合成路线图解说明: Elimination of the sulfate of (XI) with DBU in refluxing toluene led, after acidic workup, to the formation of the allylic alcohol (XII). Oxidative cleavage of the p-methoxyphenyl acetal of (XII) with DDQ produced regioselectively the p-methoxybenzoate ester (XIII). Further treatment of (XIII) with p-anisaldehyde dimethyl acetal (VII) gave the acetal (XIV). Ester hydrolysis of (XIV), followed by oxidation of the resulting alcohol (XV) with Dess-Martin periodinane afforded ketone (XVI). |
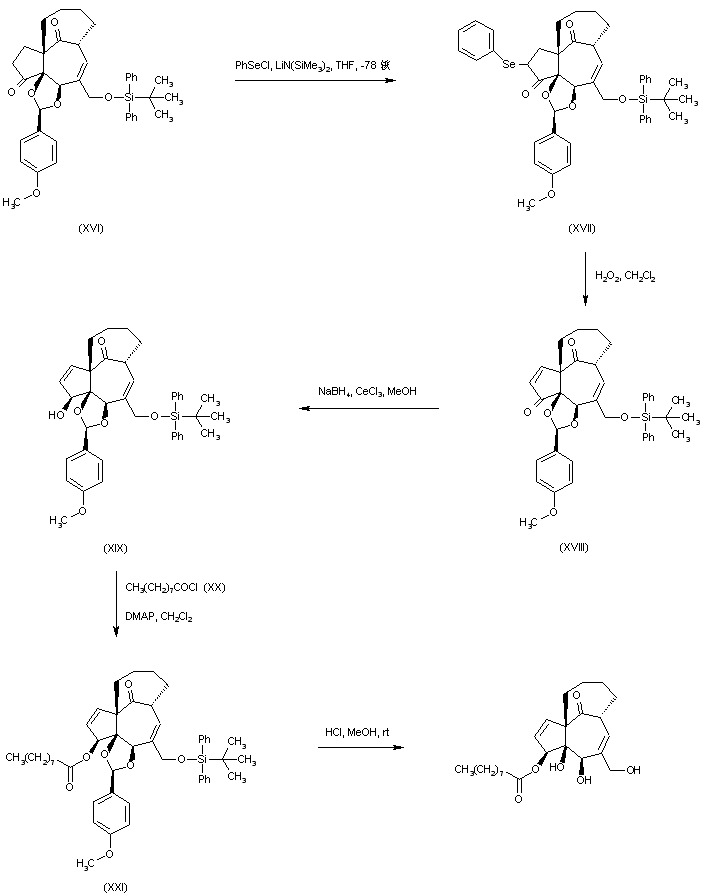 |
| 合成路线图解说明: Ketone (XVI) was treated with phenylselanyl chloride and LHMDS yielding the selenium derivative (XVII). Enone (XVIII) was then produced by oxidative elimination of the selenide (XVII). Reduction of the unsaturated ketone (XVIII) with NaBH4 in the presence of CeCl3 gave the 3-beta-allylic alcohol (XIX). This was acylated with nonanoyl chloride (XX) to afford ester (XXI). Finally, acid deprotection of the silyl ether and the p-methoxyphenyl acetal of (XXI) furnished the title compound. |