| 参考文献No. | 529150 |
| 标题: | Synthesis and biological activity of transition-state urokinase inhibitors |
| 作者: | Cohen, C.R.; Weinhouse, M.I.; Roberts, C.; et al. |
| 来源: | 217th ACS Natl Meet (March 21 1999, Anaheim) 1999,Abst MEDI 090 |
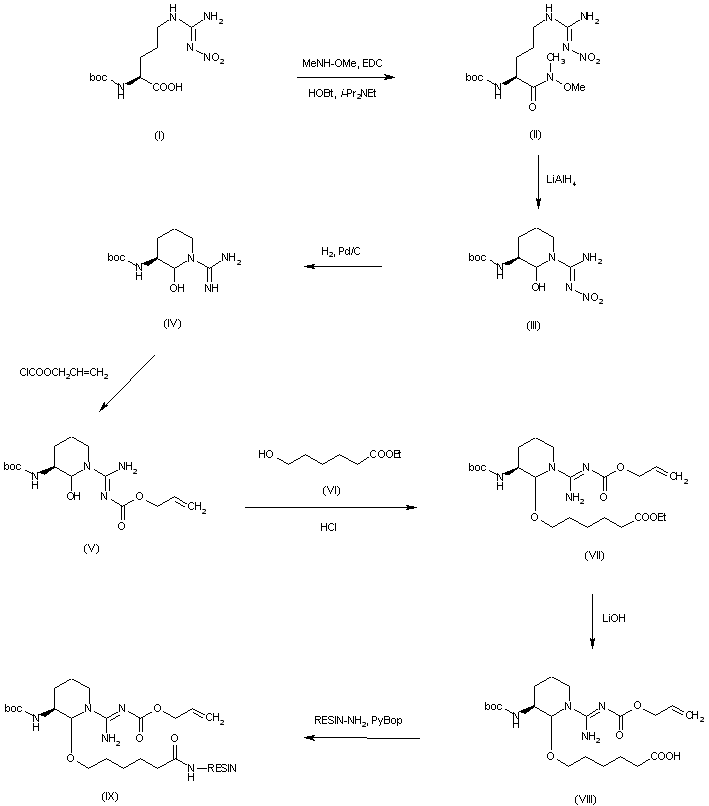 |
| 合成路线图解说明: The title compound has been prepared by either solid-phase or solution-phase synthesis. In the first case, N(alpha)-Boc-N(omega)-nitro-L-arginine (I) was coupled with N,O-dimethylhydroxylamine, and the resulting N-methoxy-amide (II) was reduced with LiAlH4 to the corresponding aldehyde, isolated as the cyclic hemiaminal (III). Deprotection of the N-nitro group of (III) by hydrogenation over Pd/C afforded guanidine (IV), which was condensed with allyl chloroformate to give the allyl carbamate (V). Subsequent acid-catalyzed condensation with ethyl 6-hydroxyhexanoate (VI) at the aminal hydroxyl group provided ketal (VII). After hydrolysis of the ethyl ester of (VII), the resulting carboxylic acid (VIII) was coupled to amino-resin to produce the amide-resin (IX). |
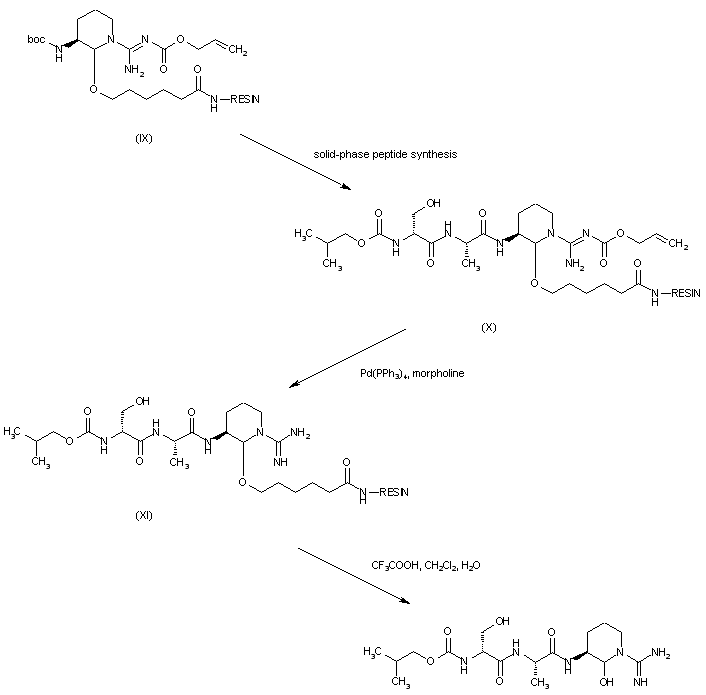 |
| 合成路线图解说明: Further solid-phase peptide synthesis by means of sequential coupling of resin (IX) with L-alanine, D-serine and isobutyl chloroformate yielded the peptide-resin (X). The allyloxycarbonyl group of (X) was then removed by treatment with morpholine and palladium catalyst to give resin (XI). The target peptide was finally liberated from the resin (XI) by treatment with trifluoroacetic acid. |
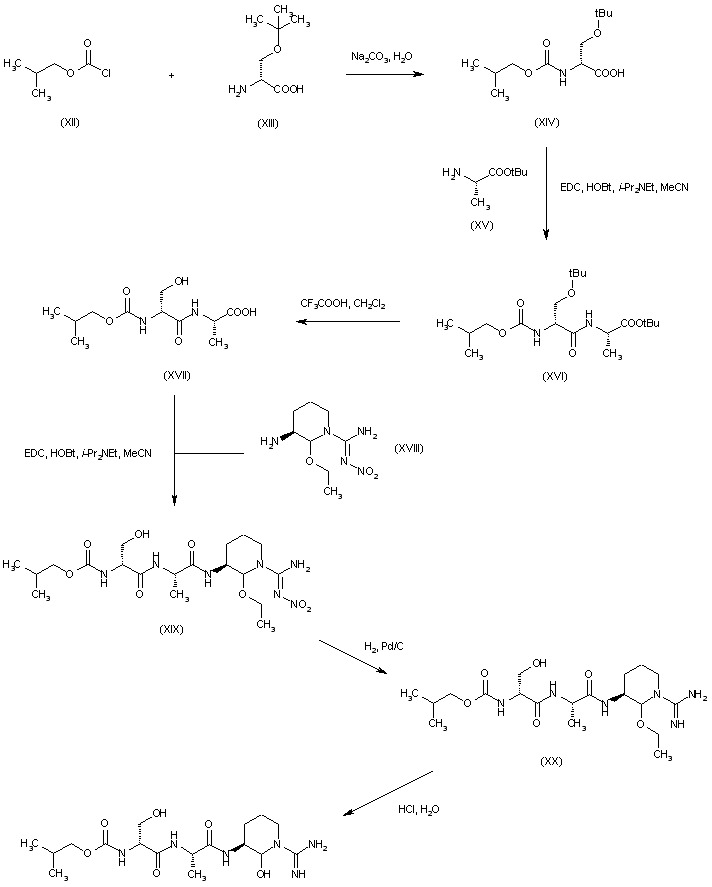 |
| 合成路线图解说明: The compound was also prepared by solution-phase synthesis. Treatment of O-tert-butyl-D-serine (XIII) with isobutyl chloroformate (XII) produced carbamate (XIV). Subsequent coupling of (XIV) with L-alanine tert-butyl ester (XV) by means of EDC and HOBt yielded the protected dipeptide (XVI). Deprotection of both tert-butyl groups of (XVI) with trifluoroacetic acid produced dipeptide (XVII), which was coupled to nitro-L-arginine ethyl aminal (XVIII) to furnish tripeptide (XIX). Hydrogenolysis of the nitro group of (XIX) over Pd/C gave (XX). The ethyl acetal of (XX) was finally hydrolyzed in 3M HCl to yield the title compound. |
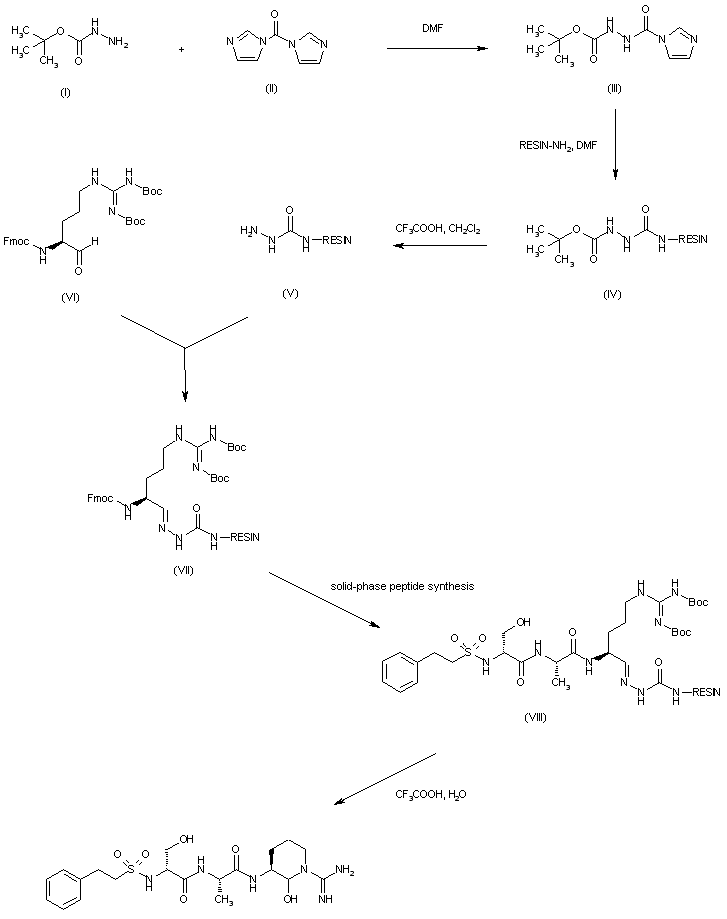 |
| 合成路线图解说明: The compound was prepared by solid-phase synthesis. Condensation of tert-butyl carbazate (I) with 1,1'-carbonyldiimidazole (II) afforded the acyl imidazole (III). Coupling of (III) with amino resin produced the Boc-protected semicarbazide-linked resin (IV), which was deprotected with trifluoroacetic acid to give (V). Condensation of (V) with protected L-argininal (VI) provided the semicarbazone resin (VII). Further solid-phase peptide synthesis by means of sequential coupling with L-alanine, D-serine and phenethylsulfonyl chloride yielded the peptide-resin (VIII). Finally, deprotection of both Boc groups of (VIII) with concomitant resin cleavage by means of aqueous trifluoroacetic acid furnished the target compound. |