| 参考文献No. | 37811 |
| 标题: | Anti-inflammatory piperazinyl-benzyl-tetrazole derivs. and intermediates thereof |
| 作者: | Maw, G.N. (Pfizer Inc.) |
| 来源: | EP 0983251; JP 2000513024; WO 9852929 |
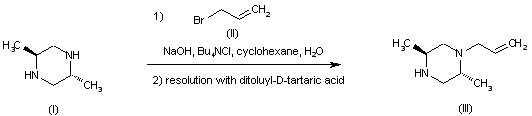 |
| 合成路线图解说明: The alkylation of the prochiral trans-2,5-dimethylpiperazine (I) with allyl bromide (II) produced a racemic mixture of N-allyl piperazines. After separation of the (+)-isomer by precipitation with (+)-camphoric acid, the desired (-)-piperazine (III) was isolated from the remaining enriched mixture by crystallization as the di-p-toluoyl-D-tartrate salt. |
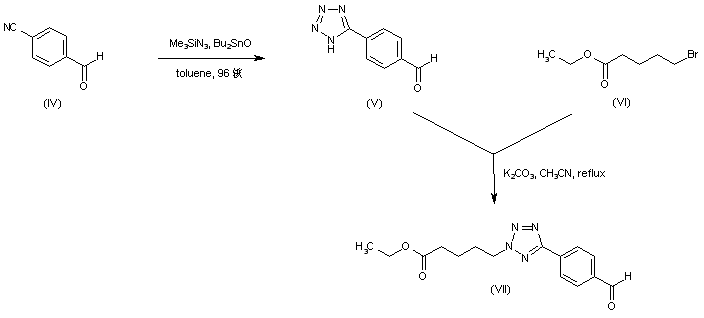 |
| 合成路线图解说明: 4-Formylbenzonitrile (IV) was converted to the aryl tetrazole (V) upon treatment with trimethylsilyl azide and dibutyltin oxide. Regioselective alkylation of tetrazole (V) with ethyl 5-bromovalerate (VI) afforded the (2-tetrazolyl)pentanoate (VII). |
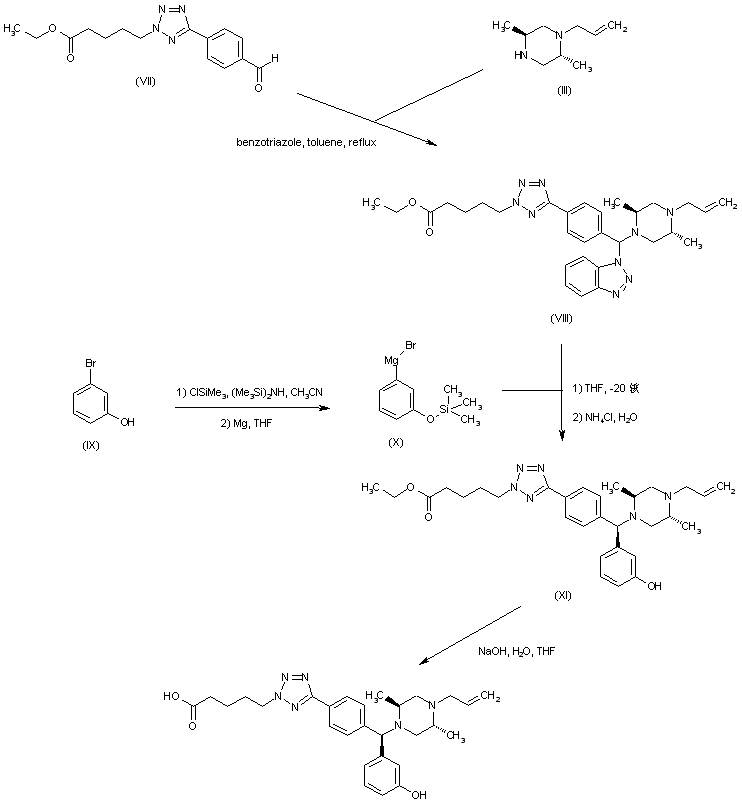 |
| 合成路线图解说明: The condensation between aldehyde (VII), piperazine (III) and benzotriazole furnished adduct (VIII). The Grignard reagent (X), prepared by silylation of 3-bromophenol (IX) followed by reaction with Mg, was then condensed with the benzotriazolyl adduct (VIII) to give the (diarylmethyl)piperazine (XI). Finally, basic hydrolysis of the ethyl ester of (XI) yielded the title compound. |
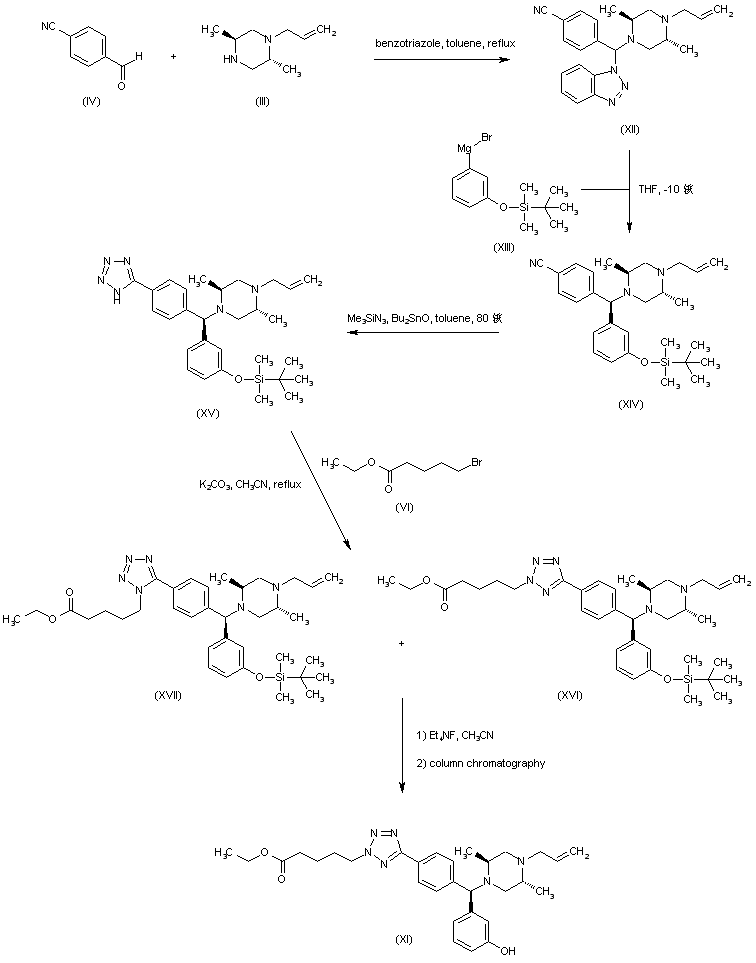 |
| 合成路线图解说明: A related procedure for the synthesis of the precursor ethyl ester (XI) was also reported. The condensation between 4-formylbenzonitrile (IV), piperazine (III) and benzotriazole furnished the benzotriazolyl adduct (XII). Subsequent reaction of (XII) with the Grignard reagent (XIII) yielded the (diarylmethyl)piperazine (XIV). The cyano group of (XIV) was then converted to the tetrazole (XV) by treatment with trimethylsilyl azide and dibutyltin oxide. Alkylation of tetrazole (XV) with ethyl 5-bromovalerate (VI) gave rise to a mixture of 1-triazolyl (XVII) and 2-triazolylpentanoate (XVI). After desilylation of this mixture with tetraethylammonium fluoride, the desired 2-triazolyl derivative (XI) was isolated by column chromatography. |